TFOS DEWS II - Sex, Gender, Hormone
David A. Sullivan, PhD1,, Eduardo M. Rocha, MD, PhD, Pasquale Aragona, MD, PhD, Janine A. Clayton, MD, Juan Ding, OD, PhD, Blanka Golebiowski, PhD, Ulrike Hampel, MD, Alison M. McDermott, PhD, Debra A. Schaumberg, ScD, OD, Sruthi Srinivasan, PhD, Piera Versura, BSD, Mark D.P. Willcox, PhD, DSc
| Download this report in PDF format |
Abstract
One of the most compelling features of dry eye disease (DED) is that it occurs more frequently in women than men. In fact, the female sex is a significant risk factor for the development of DED. This sex-related difference in DED prevalence is attributed in large part to the effects of sex steroids (e.g. androgens, estrogens), hypothalamic-pituitary hormones, glucocorticoids, insulin, insulin-like growth factor 1 and thyroid hormones, as well as to the sex chromosome complement, sex-specific autosomal factors and epigenetics (e.g. microRNAs).
In addition to sex, gender also appears to be a risk factor for DED. “Gender” and “sex” are words that are often used interchangeably, but they have distinct meanings. “Gender” refers to a person’s self-representation as a man or woman, whereas “sex” distinguishes males and females based on their biological characteristics. Both gender and sex affect DED risk, presentation of the disease, immune responses, pain, care-seeking behaviors, service utilization, and myriad other facets of eye health.
Overall, sex, gender and hormones play a major role in the regulation of ocular surface and adnexal tissues, and in the difference in DED prevalence between women and men. The purpose of this Subcommittee report is to review and critique the nature of this role, as well as to recommend areas for future research to advance our understanding of the interrelationships between sex, gender, hormones and DED.
Keywords: TFOS, DEWS II, Dry eye workshop, Dry eye disease, Sex, Gender, Hormones
One of the most compelling features of dry eye disease (DED) is that it occurs more frequently in women than men [1,2]. In fact, the female sex is a significant risk factor for the development of DED [1,2]. That such a sex-related variation exists in the prevalence of an eye disease, or any other ocular function, should not be a surprise. Sex-related differences are present in almost every cell, tissue and organ system of the body, including those associated with circulation, respiration, digestion, renal function, metabolism, and neural and endocrine activity [3]. Indeed, since 1875, more than 650,000 scientific reports have been published which address the basic and/or clinical impact of sex.
The influence of sex on the eye has been known for almost 2500 years. Conditions associated with sex differences include blepharospasm, eyelid edema, conjunctivitis, keratitis, herpetic reactivation, corneal ulcer, iritis, cataract, glaucoma, amblyopia, scotoma, optic neuritis, optic nerve atrophy and blindness [4]. As stated in a 1888 monograph addressing clinical ophthalmology “males are by no means as prone to diseases of the eye from sexual causes as females.” [5].
Since that time, investigators have identified numerous sex-related differences in the eye, and many of these variations have been attributed to the effects of sex steroids (i.e. androgens, estrogens and progestins). For example, sex-associated differences have been identified in, and sex steroids have been shown to act on, the meibomian gland, lacrimal gland, conjunctiva, cornea, anterior chamber, iris, ciliary body, lens, vitreous and retina. These hormone actions appear to be mediated through classical, and possibly membrane, receptors and impact multiple structural and functional aspects of the eye. These include tissue morphology, gene expression, protein synthesis, epithelial cell dynamics, aqueous tear output, lipid production, mucous secretion, tear film stability, blink rate and immune function [6–17]. Sex and/or sex steroids have also been linked to the development, progression and/or treatment of many ocular conditions, including DED, meibomian gland dysfunction (MGD), wound healing, keratoconjunctivitis, corneal transplant rejection and corneal pathologies [18–23].
These sex-related differences in the eye are not due solely to the effects of sex steroids. As detailed in this report, hypothalamic-pituitary hormones, glucocorticoids, insulin, insulin-like growth factor 1 and thyroid hormones also contribute notably to these sex-associated variations. In addition, sex-related differences may arise from the sex chromosome complement, including differences in parent-of-origin effects, X chromosome gene dosage (e.g. X-inactivation) and genes in the non-recombining region of the Y chromosome [24–30], as well as from sex-specific autosomal factors and epigenetics (e.g. microRNAs [miRNAs], DNA methylation and acetylation, histone modifications) [24,31,32].
It is important to note that we use the word sex for a reason. Although sex and gender are often used interchangeably throughout the literature, they have distinct meanings. As stated by the Institute of Medicine [3], sex refers to the classification of living things, generally as male or female, according to their reproductive organs and functions assigned by chromosomal complement. Gender refers to a person's self-representation as male or female, or how social institutions respond to that person based on the individual's gender presentation. Gender is rooted in biology and shaped by environment and experience. In other words, sex distinguishes males and females based on their biological characteristics. Gender, in turn, reflects socially constructed characteristics such as behaviors and expectations related to being a man, masculine, or being a woman, feminine. Furthermore, gender is dynamic, context-related and operates on a spectrum.
The correct and consistent usage of the terms sex and gender across scientific disciplines promotes the accurate assessment, measurement, and reporting of differences between men and women. In most studies of nonhuman animals the term sex should be used. The purposeful integration of considerations of sex and gender in health and disease throughout the scientific community will facilitate uptake by policy makers and dissemination to the general public.
In effect, both sex and gender affect health and disease, as well as patients' perceptions about their health. In addition, gender affects individuals' access to and interactions with the health care system. Many health disparities2 are associated with gender [33]. Eye-health disparities arise from a multitude of causes, some of which are known and some of which remain to be determined. Disparities arise from a range of influences that are biological, behavioral/perceptual, cultural, and societal. Therefore, in this report, we consider both gender and sex — terms that are distinguishable but intertwined as they both have pronounced effects on health and on health disparities. Gender and biological sex affect DED risk, presentation of the disease, immune responses, pain, care-seeking behaviors, service utilization, and myriad other facets of eye health [33].
Overall, sex, hormones and gender play a major role in the regulation of ocular surface and adnexal tissues, and in the difference in DED prevalence between women and men. The purpose of this Subcommittee report is to review and critique the nature of this role, as well as to recommend areas for future research to advance our understanding of the interrelationships between sex, gender, hormones and DED.
2.1 Does sex matter?
Sex does matter. Sex-related differences are extremely important, as they directly or indirectly influence numerous physiological and pathological functions in the body. In the past significant attention has been focused on sex-based variations at the societal and whole organism levels. However, researchers' attention towards sex-associated differences at the basic cellular and molecular levels has been inadequate [3].
To address this lack of understanding, the Institute of Medicine commissioned a six-volume report to address our knowledge of biological sex differences and to identify barriers to the conduct of research in this area [3]. The conclusions and recommendations from this report, entitled “Exploring the Biological Contributions to Human Health: Does Sex Matter,” are very relevant for our understanding, now and in the future, of sex, hormones, gender and DED.
2.1.1 Sex matters
The Institute of Medicine reported three conclusions:
• Sex (male or female) matters. Sex is an important basic human variable. Being male or female should be taken into consideration when designing and analyzing studies in all areas and at all levels of health-related and biomedical research. Individual genetic and physiological constitutions, combined with an individual's interaction with environmental factors and experiential factors, influence differences in health and illness. The occurrence, frequency and severity of diseases vary between males and females. These sex differences appear to be due to the effects of hormones, as well as other factors (e.g. genes).
• The study of sex differences is evolving into a mature science. There is now sufficient knowledge of the biological basis of sex-related differences to validate their scientific study and to permit the generation of experimental hypotheses.
• Barriers to the advancement of knowledge about sex differences in health and illness exist and must be eliminated. Scientists are confronted with a broad array of barriers when trying to conduct research on the role of sex differences in health and disease. These barriers, as summarized in Table 1 [3], encompass ethical, financial, sociological, and scientific considerations, and should be eliminated.
2.1.2 Every cell has a sex, sex begins in the womb, sex affects behavior and perception, and sex affects health
The Institute of Medicine report highlighted several findings:
• Every cell has a sex. Advances in molecular biology have identified the genetic and molecular basis of many sex-related differences in health and human disease, some of which appear due to the sexual genotype—XX in the female and XY in the male. Genes on these chromosomes can be expressed differently between males and females because of the presence of either one or two copies of the gene and because of different meiotic effects, X-chromosome inactivation, and genetic imprinting. The inheritance of either a male or female genotype is also influenced by the source (maternal or paternal) of the X chromosome. The different roles of the sex chromosome genes could explain X-chromosome-linked diseases, as well as the heterogeneous expression of some diseases within and between the sexes. There are multiple ubiquitous differences in the basic cellular biochemistries of males and females that can impact an individual's health, and these may be attributed to hormonal and genetic differences between the two sexes.
• Sex begins in the womb. Sex differences in human health and disease take place throughout the lifespan. Some originate in the intrauterine environment, others in the prenatal period, pre-puberty and puberty. Collectively, sex-related changes during these periods lay a framework for biological differences that persist through life and contribute to the variable onset and progression of disease in males and females. Consequently, it is important to research sex differences at all stages of the life cycle.
• Sex affects behavior and perception: Genetic and physiological differences, when combined with environmental factors, lead to behavioral and cognitive differences between males and females. Sex-related differences in brain organization, cognitive ability, pain perception, behavior and gender identity should be studied at all points in the lifespan. The sexual dimorphism in behavior, cognition, and perception appears to be due to hormones, genetics and other factors.
• Sex affects health. Males and females may have different patterns of illness and lifespans. Understanding the bases of these sex-related differences, as well as any similarities, is important to developing new approaches to the prevention, diagnosis, and treatment of diseases (e.g. DED).
2.1.3 Recommendations for better understanding of sex differences in health and disease
The Institute of Medicine report made a number of recommendations to advance our understanding of sex differences in health and disease. Several of these are as follows:
• Identify the roles of X- and Y-chromosome linked genes in somatic and germ-line cells, and determine with ethical research the impact of genetic sex differences on biological organization and disease susceptibility.
• Include sex as a variable in basic research, in order to reveal how sex-related differences influence health, disease and longevity.
• Select animal models for research that mirror human sex differences and are relevant for the human condition being addressed.
• Evaluate natural genetic variability, disorders of sex differentiation, reproductive status and environmental influences to gain a better understanding of human health.
• Examine sex-related differences and similarities for all human diseases that affect both sexes.
• Determine and disclose the sex of origin of cells and tissues used in biological research.
In summary, sex-related differences need to be systematically studied and elucidated, in order to advance our knowledge of their biological contributions to human health and disease. Such research is very important to permit understanding of why the female sex is a risk factor for the development of DED.
2.2 Epidemiology of sex differences in DED
2.2.1 Sex differences in prevalence and incidence of DED
Female sex is an established risk factor for DED-related autoimmune diseases such as Sjögren syndrome [34]. Female sex is also among the most widely studied and consistently identified risk factors for DED throughout the world. It is best studied in population-based epidemiological studies, since differences in care seeking behavior between women and men could influence associations in clinic-based studies (see Section 4). Among the larger epidemiological studies in North America, two parallel studies among over 39,000 women (Women's Health Study) [18] and 25,000 men (Physicians' Health Studies) [35] in the United States, showed a statistically significant age-adjusted 70% increase in risk of DED among women. Similarly, in the Beaver Dam Study of 3703 US adults, the age-adjusted prevalence of DED was significantly ∼50% higher among women (16.7% among women versus 11.4% among men) [36]. In the Beaver Dam Offspring Study, which included younger adults, the prevalence of DED was also significantly higher among women (17.9%) as compared with men (10.5%) [37]. A discrepant finding emerged from the Salisbury Eye Evaluation of over 2400 US adults aged 65 and older, in which the prevalence of one or more DED symptoms at least “often” was not significantly different at 15.6% among women versus 13.3% among men [38]. This result, combined with information from the Women's Health Study and Physicians' (men's) Health Study, suggests the possibility that the sex difference in DED may lessen with more advanced age, becoming more similar among women and men. This possibility of effect modification by age could be evaluated in existing data as well as future studies.
European studies include estimates from the Alienor Study of 915 older French adults [39], reporting approximately 60% higher prevalence of self-reported DED and over two-fold higher reported use of artificial tears among women. In the Salnes study in Spain (N = 654) [40], the prevalence of at least one of six DED symptoms at least “often” was over 70% higher among women. However using a definition of at least one symptom plus one sign, the sex difference was diminished (11.9% among women versus 9.0% among men). Nonetheless, the prevalence of a tear breakup time of ≤10 s was also higher among women in this study (17.0% versus 12.8%).
Among the now numerous studies conducted in Asian countries, most but not all have reported significantly higher prevalence of DED among women (Table 2) [41–56]. For example, Hua et al. reported in a study of 2262 Chinese adults that women were significantly more likely than men to experience at least one DED symptom at least “often” [42]. Similarly, in a separate study of 1957 Chinese adults in the Beijing Eye Study, there was a significant 56% higher adjusted risk of at least one DED symptom at least “often” among women [43]. In contrast, Lu et al. in a study of over 1800 older Tibetans at high altitude, showed a similar prevalence of DED among women and men [44]. Overall, among 17 larger epidemiological studies in Asian countries, 11 showed a higher prevalence of symptomatic DED among women than men (ranging from 16% higher to nearly three-fold higher), 2 studies showed no difference in DED prevalence, and 2 studies showed a 43%–67% higher risk among males (Table 2) [41–56].
Overall, after review of large epidemiological studies of DED, the weight of the evidence supports a generally higher risk of DED among women. Reasons for observed differences across studies could include many factors, including the definition of DED, differences in characteristics of the populations studied (such as the age-distribution), and potential differences in risk factor profiles, health-seeking behavior and health service utilization.
2.2.2 Sex differences in quality of life indicators
In addition to a generally higher risk of DED among women, in a study of 1518 women and 581 men with diagnosed DED from the Women's Health Study and Physicians' (men's) Health Study respectively, Schaumberg et al. [57] observed that women were, on average, 6 years younger at the time of DED diagnosis (mean age at diagnosis = 60 years) compared with men (mean age at diagnosis = 66 years). In addition, women reported significantly higher levels of DED symptoms as measured by Ocular Surface Disease Index (OSDI) subscale and overall scores (each p < 0.0001), as well as by The Symptom Assessment in Dry Eye questionnaire (SANDE) item and overall scores (each p < 0.0001). Severe DED symptoms on the OSDI were reported by 33.6% of women compared with 15.6% of men, whereas 39.1% of women and 17.9% of men reported severe symptoms based on SANDE [57].
In the Women's Health Study, women also reported a significantly greater impact of DED on visual quality indicators including blurred vision, poor vision, and fluctuating/unstable vision, as well as on tasks requiring sustained visual attention such as reading, driving at night, watching television, and working on a computer. Sex-related differences also extended beyond visual activities to greater feelings of depression among women, who were also less likely than men to report feeling calm and peaceful, or having a lot of energy.
2.2.3 Sex differences in burden of comorbidities
While there are now a large number of epidemiological studies that have reported on associated comorbidities [58], these studies generally did not report on the potential for sex-related differences in comorbidities. Among 3824 women from the TwinsUK cohort aged 20–87 years, the comorbid factors found to be most strongly associated with DED (highest effect sizes) included depression, pelvic pain, irritable bowel syndrome, and chronic widespread pain syndrome, and women with DED symptoms also scored significantly lower on self-perceived health [59]. In the Women's Health Study, women who used postmenopausal hormone therapy were significantly more likely to have DED (∼70% increased risk for estrogen alone, and ∼30% for estrogen in combination with progesterone/progestins) [60]. In the all-male Physicians' Health Study, reported DED-associated comorbidities included high blood pressure, benign prostatic hyperplasia and its medications, and use of antidepressants, and antihypertensives [35]. Further comparative analysis of women and men with diagnosed DED in the Women's Health Study and Physicians' (men's) Health Study showed a significantly higher comorbid burden of lupus, Sjögren syndrome, rosacea, depression, anxiety, hay fever and dry mouth symptoms among women, whereas blepharitis and meibomian gland dysfunction were reported more frequently by men [61]. Women were also more likely to use antihistamines and antidepressants, but men were more likely to use glaucoma medications.
2.2.4 Sex differences in DED treatment and treatment satisfaction
Schaumberg et al. [57] also described sex differences in treatment and treatment satisfaction. Women were significantly more likely than men to use traditional DED therapies such as artificial tears (82.8% versus 62.6%; p < 0.0001), lubricating eye ointments (19.2% versus 11.7%; p = 0.0001), and hot compresses (14.3% versus 10.7%; p = 0.02). Among treatments categorized by the Management and Therapy Subcommittee of the TFOS DEWS report [62] as Level 2 or higher, women were also significantly more likely to use oral omega-3 supplements (18.6% versus 9.6%; p = 0.0006), punctal plugs (15.0% versus 9.1%; p = 0.01), and cyclosporine ophthalmic drops (13.4% versus 6.4%; p < 0.0001). Although the majority of men and women expressed being at least “somewhat satisfied” with DED treatments, there was a significant trend for higher dissatisfaction among women with the amount of time for treatment to work (p = 0.03), and with treatment side-effects (p = 0.001); these findings were potentially related to the higher proportion of women using topical cyclosporine, for which these downsides of therapy are widely recognized [57].
2.2.5 Sex differences in the natural history of DED
In a subsample of 398 men and 386 women in the Physicians' (men's) Health Study and Women's Health Study who reported a diagnosis of DED and responded to a questionnaire about change, Leinert et al. [63] reported no significant differences by sex in reported worsening since the time of diagnosis (average 10.5 years) in ocular surface symptoms, vision-related symptoms, or the social impact of DED. However, factors associated with worsening included a previous report of severe DED symptoms, which was highly correlated with female sex. Women also had a record of more frequent corneal staining/SPK on examinations and more frequent records of results of clinical tests for DED in their medical records [63].
Other aspects of the epidemiology of DED are covered in the TFOS DEWS II Epidemiology Report [58].
2.3 Sex related differences in the ocular surface and adnexa
Significant sex-related differences have been identified in the lacrimal gland, meibomian gland, cornea, conjunctiva, nasolacrimal duct and tear film. These differences, which may contribute, in part, to the female prevalence of DED, are listed in Table 3 [6–11], [14,15,18,23,34], [64–205] and are briefly summarized below.
2.3.1 Lacrimal gland
Significant, sex-related differences exist in the anatomy, physiology and pathophysiology of the lacrimal gland (Table 3). Investigators have speculated that the increased diffuse atrophy, and orbital lobe and periductal fibrosis, present in the lacrimal glands of elderly women may decrease aqueous outflow and contribute to the sex-related prevalence of DED [193]. Additionally, the 50-fold greater expression of the asialoglycoprotein receptor (ASGPR) 1 gene in female mouse lacrimal tissue is particularly intriguing [83,89]. This receptor mediates the intracellular uptake of hepatitis C virus (HCV) [206], thereby promoting viral infection and exocrine gland inflammation [207,208]. In fact, chronic HCV infection may mimic the clinical manifestations of Sjögren syndrome [207–210] and is associated with an increased prevalence of keratoconjunctivitis sicca [211]. Another consideration is that the ASGPR is an autoantigenic target of both B- and T-cells [212]. If the ASGPR 1 gene is also upregulated in lacrimal glands of human females, and if the corresponding message is translated, this receptor expression could contribute to the increased prevalence of DED in women.
2.3.2 Meibomian gland
Sex-related differences have been identified in the morphological appearance, gene expression, neutral and polar lipid profiles, and secretory output of the meibomian gland (Table 3). Of particular interest, the sex-related differences in gene expression across species are not necessarily the same. Comparison of the 100 genes with the greatest sex-associated differences in human (e.g. lysozyme, 18.2-fold, M > F) and mouse (e.g. androgen binding protein zeta, 109-fold, F > M) meibomian glands demonstrated that none of them were the same [111]. Similarly, whereas sex was found to exert a significant impact on numerous gene ontologies and KEGG pathways, these effects were also primarily species-specific [111]. The fact that sex differences are present in the meibomian gland might be expected, given that this tissue is a large sebaceous gland and sebaceous glands are known have sex-associated differences [213]. It is possible that these sex-related differences in the meibomian gland are a factor in the development of DED.
2.3.3 Cornea
Significant sex-related differences exist in corneal anatomy and physiology (Table 3). Sex-specific changes in the cornea may also occur during the menstrual cycle, pregnancy and menopause. These alterations include variations in thickness, hydration, curvature and sensitivity, endothelial pigmentation, foreign body sensation, contact lens tolerance and visual acuity (Table 3) [214–222]. The sex-related variations in graft survival are very intriguing. While donor transplants from males have higher survival rates than from females, transplants for female recipients show higher survival rates than for male recipients [132]. This fact raises the question whether the sex of the transplant must be considered in finding the optimal transplant for the recipient. Of particular relevance to DED is the finding that the human female cornea has a significantly greater expression of the gene for transglutaminase 1 (Table 3). This enzyme catalyzes protein cross-linking and its level is typically increased in DED and corneal keratinization [223,224].
2.3.4 Conjunctiva, nasolacrimal duct and tear film
Significant sex-related differences have been identified for example in the density of goblet cells and susceptibility to inflammation (Table 3). How these variations may relate to the sex-associated prevalence of DED is unclear. Nasolacrimal ducts of females are significantly shorter and narrower than those of men (Table 3). These features, as well as the acute angle between the bony canal and nasal floor in women, may predispose to chronic inflammation of the nasolacrimal drainage system and may explain why primary nasolacrimal duct obstruction is more frequent in females [173]. Significant sex-related differences exist in the tear film (Table 3). Overall, many of these sex-related differences in the ocular surface and adnexa are likely to be due to the influence of hormones and genetics.
2.4 Sex-related differences in immunity of the ocular surface and adnexa
2.4.1 Sex and immunity
Sex differences in immune function affect both the innate and adaptive immune responses and manifest as differences in the prevalence and severity of infection and risk of developing autoimmune disorders [225,226]. In general infections are more common and more severe in males, females develop a greater antibody response and in some cases cell mediated response to vaccines but have a higher prevalence of many autoimmune diseases [225].
Examples of sex-specific differences in the innate immune response include: males have a greater percentage of pro-inflammatory cytokine producing monocytes than females [227]; females have less natural killer cell activity than males [228]; peripheral blood monocytes and plasmacytoid dendritic cells from females produce more IFNγ upon stimulation than those from males [229,230]. Examples of sex-specific differences in the adaptive response include: females have greater numbers of CD4+ve T cells and greater CD4 to CD8 ratio than males [231] and show a preponderance towards a Th2 response whereas males predominantly generate a Th1 response; in keeping with the tendency towards a Th2 response women produce greater levels of circulating antibody than men [232] including higher levels of autoantibodies when affected by autoimmune diseases [233]; in women Treg numbers vary dramatically during the menstrual cycle with a high level in the follicular phase when estrogen levels are high [234]. As alluded to with the last example, the effects of steroid hormones estrogen, progesterone and testosterone can account, at least in part, for many of the sex-based differences in immune responses. Other contributors to the observed differences are genetics, microbiome and non-biological factors.
In terms of genetic effects an obvious point of discussion is the fundamental chromosomal make up of females being XX and males XY. The X chromosome has some 1100 genes (versus the Y which harbors less than 100) including several that are involved in immune function such as certain cytokine receptor subunits and Toll-like receptors, E26 transformation-specific domain-containing protein Elk-1 which is involved in B cell development and FOXP3 which is important for Treg development [225]. Early in development one of the X chromosomes in a female is transcriptionally silenced in a random process such that in some cells it is the maternal X that is “turned off” and in others it is the paternal X. In this way mutations/polymorphisms in X-linked genes that may affect the immune response can be minimized in females whereas in males the effects of the altered gene will be manifest. In humans approximately 15% of the genes on the inactive X chromosome actually remain active thus it is also possible for females to have increased expression of some X-linked genes if both copies have remained active [235]. Depending on the gene involved this may contribute to enhanced immune responses. Interestingly, males with Klinefelter syndrome, where there is an additional copy of the X chromosome, show some immunological features, such as antibody levels, more similar to females than a normal XY male [236,237]. Further they have a 14-fold increase in the prevalence of lupus compared to XY males, with a similar risk for developing the disease as females [235].
Attention has been drawn to the potential role of epigenetic regulation by microRNAs (miRNAs) in sex-related immune differences. MicroRNAs are small double-stranded non-coding RNAs that negatively regulate gene expression by translational repression or mRNA destruction. Some 800 miRNAs have been identified in humans with approximately 10% being located on the X chromosome [238]. Several X-linked miRNAs are involved in immunity including miR-98 which regulates TLR mediated responses, miR-223 which negatively regulates granulocyte maturation and miR-503 and -542 which regulate monocyte differentiation [238,239]. It has been documented that miRNAs are differentially expressed among males and females in many tissues leading to the proposal that their differential expression in immune cells will also contribute to sex-related differences in immunity and susceptibility to autoimmunity [239]. Interestingly it was reported that several X-linked miRNAs were overexpressed in T cells from female lupus patients compared to males [240].
Another factor that may contribute to sex-differences in immunity are the microbial communities all humans harbor. Microbes, primarily in the intestine but also at other extra-intestinal niches, have been recognized to have an essential role in the development, maturation and modulation of the host immune response, [241]. Hormonal status can affect the composition of the microbiome in a sex specific manner while in turn members of the microbial community can metabolize sex hormones so influencing their effects on host immunity [242]. The existence of this relationship was shown in studies where manipulation of the microbiome conferred protection from autoimmune diabetes in female non-obese diabetic (NOD) mice in a sex hormone dependent manner [243,244]. Sex-related non-biological factors may also influence immunity [245]. For example some immunomodulators such as chemicals and metals produce work hazards that affect mostly males as they are the predominant sex working in that environment owing to the specific physical characteristics needed to perform the work, and also to gender norms for behavior.
2.4.2 Influence of sex on ocular surface and adnexal immunity
The ocular surface and adnexa mount a very robust immune response to ensure the health of the eye and maintenance of good vision. The lacrimal gland contains a diverse array of T cells, B cells and their fully differentiated Ig secreting form – plasma cells, as well as dendritic cells and macrophages. The conjunctiva hosts secondary lymphoid tissue, called conjunctival associated lymphoid tissue and both cornea and conjunctiva are endowed with antigen presenting cells for rapidly responding to ocular surface antigens. Corneal and conjunctival epithelial cells also can respond to various antigens and synthesize and secrete many proteins that are important in innate and adaptive immune responses [246,247].
Despite the long recognized sex-bias in general immunity (see Section 2.4.1) very few studies have examined sex-related differences in the ocular immune response per se. The tear film is an essential component of the ocular immune response and contains many components with antimicrobial functions [248] and a small number of studies have compared the levels of some of these antimicrobial components in males and females. Secretory IgA at the ocular surface binds and neutralizes pathogens and facilitates their removal. Although females produce higher levels of antibody than males [232] most studies have found no differences in the concentration of IgA in tears in humans [249–252] A prominent sex-related difference has however been observed in rats where IgA and free secretory component were consistently higher in the tears of adult male rats than females [100,103]. Further, there was greater secretion of secretory component by the lacrimal tissue and a greater density of IgA positive cells in the lacrimal glands from male compared to female rats [75,102]. These effects appeared to relate to androgen function (see Section 3.1). Most studies show no sex-specific differences in tear levels of lactoferrin, lysozyme or phospholipase A2, all of which are antibacterial proteins [251–256]. In contrast, at least in rabbits, the concentration of lipocalin was increased in adult male rabbits in lacrimal fluid and lacrimal gland [98].
The density of corneal dendritic cells was not different in male and female contact lens wearers [257], although female mice on a C57BL/10 background lacking the capacity to produce γδ T cells had a much greater incidence of spontaneous keratitis than males [258]. These findings suggest that, at least in animals, some of the immune cells that act as a bridge between the innate and adaptive immune responses may be involved in sex-specific responses. In terms of the adaptive response, it has been observed that male and female rats have different patterns of T cell presence in the lacrimal gland over their lifetime [70,259]. Most studies have demonstrated that humans have no sex-related differences in lacrimal lymphocyte infiltration [195,259,260].
Thus, overall the preponderance of studies suggests few differences amongst the ocular surface/adnexal immune response in males and females in humans, at least in the absence of overt disease. However, it may be that the differences in immunity in males and females primarily manifest when the immune system is challenged. Thus, male sex has been found to be risk factor for developing keratitis with contact lens wear in some studies [261,262] (although the effect of gender behavior rather than sex might also explain this difference), and corneal re-epithelialization after development of fungal-related corneal ulcer took twice as long in females than males [149].
Sex-specific differences have been observed in some animal models of autoimmune DED, for example in the MRL/lpr mouse dacryoadenitis is significantly more severe in females than males whereas in NOD mice lacrimal gland pathology and lymphocyte infiltration was much greater in male than female mice [64,65,263]. How sex-related differences in immunity contribute to these observed variations has not been widely studied but recently it was observed that Treg dysfunction contributes to the male-bias seen in the NOD mice [264]. A study also addressed sex-specific differences in a desiccating stress model of DED [265]. Female mice exhibited more severe DED signs having increased corneal epithelial defects, decreased conjunctival goblet cells and lower production of mucin and tears compared to male mice. Interestingly the number of neutrophils in the lacrimal glands and draining lymph nodes of normal (non DED) mice was 2–4 times greater in females than in males. However, when experimental DED was induced in the animals the numbers of these neutrophils increased 2–12 fold in males but was decreased in females. Furthermore, the authors found that in females, as the neutrophils decreased there were increases in Th1 and Th17 cells and a decrease in Treg cells, leading them to suggest the neutrophils were acting as suppressor cells, with neutrophil specific production of lipoxin A4 mediating the effect [265].
2.5 Sex, gender, and the pain of DED
Pain is a difficult word to define, and many definitions have been proposed. “Whatever the experiencing person says it is, existing whenever s/he says it does” emphasizes the subjective experience of pain with no objective measures [266]. The latest and widely accepted definition of pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”, which emphasizes that pain is a complex experience with multiple levels involved [267]. Detailed information related to the pathology of pain in DED is reported in the TFOS DEWS II Pain and Sensation Report [268], including pathways carrying pain signals to the brain, brain areas involved in pain perception, and the location, characterization and role of pain receptors in the lacrimal functional unit. Evidence in the literature on sex-related differences in pain is mostly not specific for the ocular surface. Relatively little attention has been paid to the relationships between sex and pain in DED as yet, but the current research on this issue is reported in this section.
Pain is common. The worldwide prevalence of chronic pain (defined as the pain that extends 3 or 6 months beyond onset or beyond the expected period of healing) is 25–30% [269–271], and is greatest in those affected by heart disease, cancer, and diabetes [272]. About a fifth of those who report chronic pain are thought to have predominantly neuropathic pain [269]. According to recent hypotheses, chronic pain in DED may also be a neuropathic pain [273,274]. It is known that female sex and older age are main factors associated with chronic pain [275]. The higher incidence of pain-related symptoms among women compared with men has been ascribed to sociocultural (gender-related) factors or biased reporting. However, sex differences in experimental pain response in animal or human studies and the higher prevalence in females of chronic pain syndromes [276] would suggest an underlying biological mechanism that is sex-related [277].
There are some common misconceptions about pain that have hindered the investigation of pain mechanisms and sex-related differences, in general and also specifically in DED. Some of the most popular incorrect beliefs include that i) pain does not exist in the absence of physical or behavioral signs or detectable tissue damage; ii) pain without an obvious physical cause is usually psychogenic; and iii) patients who respond to a placebo drug are malingering [266].
In DED, pain is difficult to describe due to the multifactorial nature and chronicity of the disease. Patients describe their sensations with a variety of expressions (dry/dryness, gritty, burn/burning hot, red, crust, shut, discomfort, visual changes, sore-irritated, gritty-scratchy, foreign body/foreign body sensation, burning, light sensitivity, itching, irritated, feeling of watery eyes, sharp, cutting, needle-like, pins and needles, pounding, pressure/aching) [18,274,278–281], so it may be difficult to correlate different descriptions with pain type and severity. Sex differences in reporting symptoms in DED have been found in a large epidemiological study [57]. Women with DED reported significantly greater problems with vision, reading, driving at night, watching television, and working on a computer compared to men with DED, as measured with OSDI subscales. This finding points primarily to perceived difficulties with visual tasks, but the tools for specifically measuring pain sensations (such as those described in 4.5.2) were not utilized in this survey.
A correlation also exists between DED and chronic pain as comorbidity [282,283]. Patients with non-ocular chronic pain diagnoses were more likely to carry a DED diagnosis compared with their counterparts without chronic pain. Fibromyalgia, a female prevalent disease, is now believed to be a brain disorder characterized by aberrant central pain facilitation and a state of hyperalgesia which may be due to impaired descending inhibition. Symptoms of fibromyalgia are often associated with DED symptoms [284], as are other conditions including chronic fatigue syndrome and sleep disorders.
2.5.1 Sex differences in pain assessment
A reliable evaluation of a possible sex difference in subjective reporting of pain in DED remains lacking. Commonly utilized and rather easy to use, one-dimensional pain assessment tools include the Numeric Rating Scale (NRS), the Visual Analogue Scale (VAS), and the Faces Pain Scale (FPS) [285]. In DED literature these three scales have been described [286,287] yet seldom utilized in studies. In fact, the OSDI or other validated subjective symptom questionnaires are more frequently utilized, with the aim to score a sum of symptoms rather than pain intensity. To cover and quantify the multiple aspects of pain intensity and disability, multidimensional assessment tools are also used and include the Brief Pain Inventory (BPI) and the McGill Pain Questionnaire (MPQ), the latter focusing on assessment of sensory and affective dimensions of pain [285]. In DED literature, use of these tools, albeit limited, has just been reported [288].
2.5.2 Sex differences in ocular surface sensitivity
As extensively reported in the TFOS DEWS II Pain and Sensation Report [268], functional types of corneal sensory receptors have been demonstrated and mapped [289]. Sex-related differences in ocular surface sensitivity are equivocal [140,290–292]. Premenopausal women have been found to be more sensitive to corneal stimulation than men of similar age, but overall there were no differences in mechanical and chemical thresholds between men and women [141]. High pain sensitivity and low pain tolerance, assessed with quantitative sensory testing using heat stimulus on the forearm, were found to be associated with symptoms of DED in female twin volunteers from the TwinsUK adult registry [293,294].
2.5.3 Sex differences in general pain sensitivity, tolerance, intensity and unpleasantness
The current knowledge on chronic pain mechanisms involves complex brain circuits that include sensory, emotional, cognitive and interoceptive processing [295]. The neural networks join physiological systems (such as sensory, immune, endocrine, autonomic, motor systems and the sleep-wake rhythm) and psychological systems (such as perception, motivation, emotion, cognition, attention and memory) to behaviors [295]. It is difficult to draw firm conclusions as to sex influences in such complex interconnections. Various population-based studies suggested that women were more likely than men to experience a variety of chronic pain syndromes [296–299], and tend to report more severe pain [300], at a higher frequency and in a greater number of body regions [301]. However, results from reviewed literature were not always consistent and were affected by numerous confounding variables. Table 4 [302] presents a summary of data on the major outcomes from the literature for most pain modalities tested in multiple anatomical regions in the laboratory setting [297].
2.5.4 Pain perception in DED subjects
As already reported in the TFOS DEWS II Pain and Sensation Report [268], experimentally induced pain perception in DED in humans can be assessed in the laboratory. Stimuli can be delivered to subjects by using 1. the controlled adverse environment (CAE) model [303–305], which induces DED signs and symptoms by regulating humidity, temperature, airflow, lighting conditions and visual tasking; 2. The Cochet-Bonnet esthesiometer, a handheld instrument in which a nylon filament is extruded, a calibrated scale provided by manufacturer is then used to convert filament length measurements to pressure. The instrument evaluates corneal and conjunctival sensitivity to mechanical pressure but has some limitations [306]; 3. The Belmonte Gas esthesiometer [307,308], and its modified version [309] which uses a jet of air to estimate ocular surface sensitivity to mechanical, chemical and thermal stimuli.
Sex was included as a variable playing a role in sensitivity thresholds in one study [308], where chemical sensitivity was found to be significantly lower in men than in women, whereas no significant differences were identified for the other stimuli. Another study [310] utilized the Cochet-Bonnet esthesiometer and found that corneal sensitivity was higher in men than in women, but only in superior, temporal and inferior areas. No sex-related differences were found in the corneal sensitivity response to cold stimuli induced by tear film evaporation during sustained eye opening in normal subjects or DED patients [311]. Conjunctival sensitivity, like that of the cornea, is higher in females than males with a trend towards an age-related increase in females, which is not apparent in males [140]. A study utilizing a different esthesiometer reported higher sensitivity of the cornea (and conjunctiva) in females compared to males, with an age-related increase apparent in females only [140].
2.5.5 Sex differences in pain and the role of bio-psycho-social factors
Bio-psycho-social factors, include hormonal factors exposure to sex steroid hormones (biological factors), blood pressure, heart rate, peripheral and central processing of the stimuli (physiological factors), genotype, depression, anxiety/stress, coping, believing that something is worse than it is (psychological factors), and gender role expectations, experimenter sex/gender, past history of pain (social factors).
Extensive studies have investigated the role of endogenous or exogenously administered sex steroid hormones on pain sensitivity and perception. For a comprehensive review, readers are referred to Bartley and Fillingim [298]. Due to conceptual deficits such as small sample sizes, experimental session timing across the menstrual cycle and lack of biological markers to stage the cycle (such as urine or blood sample testing), the effects of estrogens on pain responses have been found to be inconsistent, minimal, or absent [298]. The role of androgen has been understudied [298]. Ocular discomfort (OSDI score) was higher during ovulation as compared to the luteal phase of the menstrual cycle [312,313]. Phytoestrogen or dehydroepiandrosterone (DHEA) supplementation was found to be associated with reduction in discomfort symptoms [314,315]. A weak correlation between higher levels of androstenedione and subjective symptoms have been found [316]. (see also Section 3.1).
Physiological factors related to the nervous system and in particular to peripheral sensitization, primary hyperalgesia and central processing nociception, and allodynia are discussed in the TFOS DEWS II Pain and Sensation Report [268]. Here it was noted that methodologies employed to investigate the issue were not applied in DED in general or in sex-related differences specifically. Although phasic pupil dilation as a physiological marker of preconscious brain activity, brain activation imaging by positron emission tomography, and brain functional magnetic resonance imaging (fMRI) have failed to demonstrate clear sex differences in responses. There are clearly insufficient brain imaging data to draw firm conclusions that go beyond speculation, and more extensive research with positron emission tomography and fMRI is needed [298].
The role of genotype in pain is still understudied but some evidence is now emerging in terms of sex-related differences [276,317]. For instance, the melanocortin-1 receptor (MC1R) gene, associated with red hair and fair skin, has been found to moderate analgesia in a sex-dependent manner [317]. In DED, polymorphisms in the proinflammatory cytokine genes IL-1β (rs1143634) and IL-6R (rs8192284) were reported to be associated with non-Sjögren syndrome DED symptoms in a Korean population [318]. Chronic pain syndromes, including DED, have been reported to display a heritable component, as shown in a large twin-cohort population study [319] but a sex/gender-related difference was not shown.
2.5.6 Sex differences in pain and the role of psychological factors
Inconsistent or contradictory results were obtained with regard to the direction of the association between anxiety/depression with sex and across outcome measures. Acute pain induces depressed mood [320] and chronic pain is known to cause depression [321]. Pain and depression are important comorbidities as both clinical and preclinical studies clearly indicate that pain is depressing, and depression can cause and intensify pain. But what comes first, and does a measure exist to objectively quantitate and time these events? [320,321] Sex differences in the prevalence of depression are widely established as depression is more common among females (21%) than males (13%) [322], although this might be confounded by gender differences in reporting depression or seeking treatment. Post-traumatic stress disorders are conditions that frequently coexist with chronic pain [323]. Post-traumatic stress disorders were more common in male veterans with DED than in those without and that male veterans with a DED diagnosis had a twofold higher risk of carrying a diagnosis of depression [324–326]. DED symptoms were more closely aligned to non-ocular pain, depression and post-traumatic stress disorders than to tear film parameters [327]. In female populations, depression, stress and DED symptoms were also closely correlated [59,328]. Of course no sex difference could be retrieved from any of these papers.
The associations between depression, mood disorders, anxiety conditions and the severity of the symptoms in DED have been discussed in a case study [329]. The prevalence of sleep and mood disorders was found to be significantly higher in patients with DED and in correlation with age but not with sex [330]. In addition, depressive symptoms were found to be associated with DED symptoms [329,331–335] but no sex-related differences were reported in large population-based studies [336]. Taken from another perspective, a higher level of subjective happiness, as measured by a validated score [337], was inversely and significantly related to self-reported DED symptoms in a VDT users population but a sex-related difference was not found as well [338].
Other important psychological factors involve pain catastrophizing, a coping style which connotes negative emotional thoughts toward pain and adapting coping strategies [299]. In this respect, it is suggested that women tend to cope better with pain when they employ pain attentional focus or reinterpret pain sensation, whereas distraction may be more efficient in men [339–341]. None of these factors has been considered in DED papers as regards possible sex differences.
2.5.7 Gender differences in pain and the role of social factors
Gender role broadly refers to a socially accepted set of characteristics ascribed to each sex. With regard to pain, the feminine role is stereotypically associated with greater willingness to report pain, whereas the expected masculine role is more related to stoicism. A measure of gender-related personality traits (masculinity–femininity) is given with the Bem Sex Role Inventory [342]. The emotional vulnerability related to the masculinity-femininity trait and the perceived identification according to typical male/female stereotypes seem to alter pain tolerance, intensity, and unpleasantness [343]. However, none of these social factors were considered in DED papers related to sex differences. Subjects perform better (i.e. higher pain tolerance or lower mean pain intensity) on a laboratory pain task when they are tested by an experimenter of the opposite sex [344]. Past history may influence pain perception in women but not in men. However, the literature needs to be enriched before drawing any strong conclusion. Finally, ethnic and cultural aspects represent an important but yet understudied issue [345,346]. None of these factors have been considered as regard sex-differences in DED pain [347].
In conclusion, information on the relationship among DED, sex and the perception of pain is still not conclusive or unequivocal. Knowledge on possibly different sex-related mechanisms for pain process in DED is still limited. Sex differences in the response to experimentally induced ocular surface pain in healthy subjects remain understudied. Laboratory studies on sex-related differences in pain perception should be performed on healthy volunteers of various ages and on patients with painful pathologies (primary and secondary outcomes defined beforehand, sample size estimated as a function of clinical significance). The application of validated pain assessment tools in the clinic is still limited (only restricted to clinical trials) and standardized and more uniform testing procedures need to be adopted. The use of promising neuroimaging techniques is still very limited. All these points may represent the basis and suggestions for future studies.
The endocrine system plays a significant role in the regulation of, and the sex-related differences in, the ocular surface and adnexa. Hormones from this system are also implicated in the development and/or treatment of aqueous-deficient and evaporative DED. These hormones include androgens, estrogens, progestins, hypothalamic-pituitary hormones, glucocorticoids, insulin, insulin like growth factor 1 (IGF-1) and thyroid hormones. This section reviews the relevant actions of these hormones and their involvement in DED.
3.1 Androgen regulation of the ocular surface and adnexa
Androgens are extremely important in the regulation of the ocular surface and adnexa [20,348–350]. They also appear to mediate many of the sex-related differences in these tissues [83,90,351–353]. Conversely, androgen deficiency is associated with both aqueous-deficient and evaporative DED [20,316,348–350,354–356]. In fact, as shown in an extensive metabolomics study of 390 different plasma metabolites in 1622 women with DED, unusual androgen metabolites (e.g. epiandrosterone, a hormone with weak androgenic activity) are key biomarkers for DED [357]. The impact of androgens on ocular surface and adnexal tissues is summarized below.
3.1.1 Androgen regulation of the lacrimal gland
3.1.1.1 Androgen influence and mechanism of action
The lacrimal gland is an androgen target organ. Androgens exert a considerable impact on the structure and function of this tissue, including its cellular architecture, gene expression, protein synthesis, immune activity, and fluid and protein secretion [11,65,66,74,78,79,84,86,88,91,96,104,105,204,358–390]. Androgen deficiency, in turn, has been linked to lacrimal gland dysfunction and a corresponding aqueous tear deficiency [35,348–350,356,369,391–393].
Insight into the magnitude and extent of androgen influence on lacrimal tissue may be gained by reviewing the effects of orchiectomy and/or androgen replacement therapy on this gland. As shown in Table 5 [11–13,35,66,74,77–79,84,86–88,91,93,94,96,100,102–105,199,204,358–373,375–413], investigators have reported that castration, or exposure to androgen receptor antagonists, significantly impair lacrimal gland anatomy and physiology. Alterations include degenerative changes, such as reduced growth and activity, loss of glandular elements, an attenuation in acinar cell size, a decrease in nuclear volume and polymorphism, proliferation of connective tissue, disruptions in protein levels, changes in enzyme activity, alterations in fluid and protein secretion, and a transformation of the gland's morphological appearance into a neutral or ‘female’ type. Conversely, researchers have also reported that androgen replacement therapy reverses the impact of castration, and may lead to profound changes in tissue structure, cellular activity and glandular secretion. These alterations may include acinar epithelial cell hyperactivity, the appearance of abundant glycoprotein secreting cells, an enlargement of glandular vesicles, the generation of mucus and highly polymerized carbohydrates, the suppression of inflammation, an evolution of the glandular acino-serous structure into a “vesicular mucus” pattern, and a change in tear protein and fluid secretion (Table 5).
Many of these androgen-induced effects have a molecular biological basis. As shown in studies with mice, androgens modulate the expression of thousands of lacrimal gland genes involved in biological processes, molecular functions and cellular components [90,351]. Gene ontologies most affected by testosterone include those associated with cell growth, proliferation and metabolism, cell communication and transport, nucleic acid binding, signal transduction and receptor activities [90,351]. Indeed, some of the most significant androgen actions are directed towards the stimulation of mitotic cycles, DNA metabolic processes and chromosomal components, and these responses may underlie androgen's ability to promote epithelial cell proliferation as seen in the rabbit [414,415], and a rise in tissue weight as seen in the mouse [358,384].
Numerous androgen derivatives influence lacrimal processes, such as the suppression of glandular inflammation in mouse models of Sjögren syndrome (see below example [66,91,97, 100,102,103,204,367,372,374−377,380,412] These hormones include: [a] testosterone derivatives (e.g. testosterone, 19-nortestosterone, methyltestosterone and fluoxymesterone); [b] 4, 5α-dihydrotestosterone derivatives (e.g. dihydrotestosterone (DHT), methyldihydrotestosterone, oxymetholone, 5α-androstan-17α-ol-3-one-acetate, 5α-androstan-17β-ol, stanozolol and 5α-androstan-2α-methyl-17β-ol-3-one); [c] 17β-hydroxy-5α-androstane derivatives containing a ring A unsaturation, and excluding testosterone derivatives (e.g. 2, (5α)-androsten-17β-ol); [d] 19-nortestosterone derivatives (i.e. 19-nortestosterone, 19-nortestosterone propionate); [e] 4-estren-7α-methyl-17β-ol-3-one; [f] androgenic compounds with unusual structural features (i.e. oxandrolone and 5α-androstan-17β-ol-3-oxime, which contains a nitrogen derivative substitution for the 3-ketone function in dihydrotestosterone); [g] adrenal cortical androgens (i.e. dehydroepiandrosterone, an androgen precursor, as well as expressing androgen action).
Androgen effects on the lacrimal gland may be enhanced or attenuated by a variety of neurotransmitters, cytokines, secretagogues, autocoids, hormones, factors and viruses. Modulatory factors include vasoactive intestinal peptide, β-adrenergic agonists (e.g. isoproterenol), cholinergic agonists (e.g. carbachol), IL-1α, IL-1β, tumor necrosis factor-α, cyclic AMP analogues (e.g. 8-bromoadenosine 3’:5’-cyclic monophosphate), cyclic AMP inducers (e.g. cholera toxin and prostaglandin E2), phosphodiesterase inhibitors (e.g. 3-isobutyl-1-methylxanthine), pertussis toxin, insulin, glucocorticoids, cyproterone acetate, retinoic acid, prolactin, extracellular calcium, high-density lipoprotein, epidermal growth factor (EGF), fibroblast growth factor, putrescine, sialodacryoadenitis virus, cytomegalovirus, and factors from the pituitary, thyroid and adrenal glands [13,74,91,360,364,366,367,378,388,389,412,414].
The primary mechanism by which androgens act on the lacrimal gland appears to involve binding to saturable, high-affinity and steroid-specific receptors in acinar and ductal epithelial cells. These “classical” androgen receptors are members of the nuclear receptor superfamily of ligand-inducible transcription factors and appear to mediate most of the “classical” actions of androgens [418,419]. The location of androgen receptor protein is generally intranuclear, due to the presence of a nuclear targeting signal, similar to that of the SV 40 large T antigen, which occurs in the receptor hinge region immediately following the DNA-binding domain [420]. After androgen binding to the androgen receptor, the monomeric, activated hormone-receptor complex associates with androgen response elements in the regulatory region of specific target genes and, in combination with appropriate co-activators and enhancers, modulates gene transcription, protein synthesis and tissue function [418,419,421–424].
In support of this mechanism of action are the observations that: (a) androgen receptor mRNA is expressed in lacrimal glands of mice, rats, hamsters, guinea pigs, rabbits and humans [352,389,414,425,426]; (b) androgen receptor protein is present predominantly within epithelial cell nuclei of lacrimal tissues of mice, rats, hamsters and humans [97,372,385,389,427,428]; (c) lacrimal glands feature a single class of saturable, high-affinity and steroid-specific androgen binding sites, which have a dissociation constant and stereochemical selectivity similar to those found in many cells and tissues throughout the body [425,429]; (d) androgen-androgen receptor complexes in lacrimal tissue associate with DNA [429]; (e) androgen effects in lacrimal glands or in isolated acinar epithelial cells may be curbed by antagonists of, or mutations within, androgen receptors, as well as by inhibitors of transcription and translation [88,102,199,367,414]; (f) androgens, as noted above, exert a significant influence on gene expression and protein synthesis in lacrimal glands [11,13,74,77,79,86–88,91,97,100,102,104,105,352,353,359,363,364,366,371–373,378,381–383,386,388,389]. Androgens also modulate the expression of their own androgen receptors in the lacrimal gland by increasing the content of androgen receptor protein and decreasing the level of androgen receptor mRNA [97,372,389,430]. This form of autoregulation also occurs in other, but not all, androgen target organs [431–435].
In addition to nuclear androgen receptors, androgens may possibly act on the lacrimal gland through nonclassical pathways. These pathways, which typically occur within seconds to minutes, involve hormonal interaction with stereospecific plasma membrane receptors and lead to rapid changes in membrane fluidity, the activity of neurotransmitter receptors and/or the control of transcription factors [424,436–442]. However, evidence for such lacrimal membrane receptors has yet to be obtained. Androgen-binding proteins have also been identified in lacrimal glands and tears of male and female mice, but not yet examined in humans [443–445]. The role of androgen-binding proteins is unknown.
The source of androgens that act on the human lacrimal gland may primarily be from local, intracrine synthesis (Fig. 1). As demonstrated in the field of intracrinology, the vast majority of androgens in women (i.e. most before and all after menopause), and a significant percentage in men (e.g. 40–50%), are synthesized in peripheral tissues from adrenal sex steroid precursors (i.e. DHEA, DHEA-sulfate [DHEA-S] and androstenedione) [446–458]. In fact, humans and primates are unique in possessing adrenal glands that secrete large amounts of DHEA and DHEA-S, which are then converted into androgens (e.g. testosterone, DHT) and estrogens by steroidogenic enzymes in peripheral sites and allow target tissues to adjust the formation and metabolism of sex steroids to local requirements [449,453]. Human lacrimal glands produce mRNA for Type 1 and 2 5α-reductase [427], and mRNAs for steroid sulfatase, 3β-hydroxysteroid dehydrogenase (HSD)-Δ-5Δ4-isomerase type 1, 17β-hydroxysteroid dehydrogenase types 1 and 3, aromatase, glucuronosyltransferase and sulfotransferase [459].
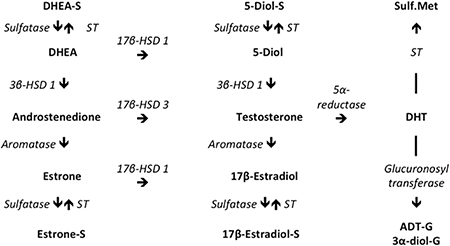
Fig. 1 Major metabolic pathways of androgens and estrogens in humans. The directions of enzymatic reactions are shown by arrows. Abbreviations are 17β-HSD 1, 17β-HSD 3 = 17β-hydroxysteroid dehydrogenases types 1 or 3; Sulfatase = steroid sulfatase; 3β-HSD 1 = 3β-hydroxysteroid dehydrogenase (HSD)-Δ-5Δ4-isomerase type 1; ST = sulfotransferase; Sulf.Met = sulfated metabolites; HSD = hydroxysteroid dehydrogenase; DHEA = dehydroepiandrosterone; DHEA-S = DHEA sulfate; Estrone S = estrone sulfate; DHT = dihydrotestosterone; 5-diol = 5-androstene-3β,17β-diol; ADT-G = androsterone-glucuronide; 3α-diol-G = androstane-3α,17β-diol-glucuronide. Modified and reprinted with permission from Wolters Kluwer Health, Inc [459].
The presence of aromatase in the lacrimal gland raises the question as to whether many of the androgen actions on the lacrimal gland might possibly be mediated through its aromatization to estrogens. Aromatase is an enzyme that catalyzes the transformation of testosterone and androstenedione to 17β-estradiol and estrone, respectively. The answer is no. Less than 2% of the genes upregulated by testosterone in lacrimal tissues of orchiectomized and ovariectomized mice are also increased by 17β-estradiol [90]. Moreover, of those genes influenced by both testosterone and 17β-estradiol in lacrimal glands of castrated female mice, over 60% of the sex steroid effects are in the opposite direction [90]. Thus, androgen action on the lacrimal gland is not mediated primarily through a conversion to estrogens.
The effects of androgens on lacrimal gland structure are not the same in all species [204]. For example, androgen administration increases the acinar epithelial cell area and lacrimal gland weight/body weight (LGW/BW) ratio in intact female mice [358,374,376,384], but rarely alters these variables in lacrimal glands of castrated male or female rats or rabbits, and may even reduce the LGW/BW ratio in guinea pigs [204]. Further, androgens promote the proliferation of rabbit acinar epithelial cells in vitro, [414,415] but do not induce such an effect on rat lacrimal gland cells in culture [364]. Consequently, although androgen action on lacrimal gland structure is considerable, it is unlike that of the ventral prostate, which in most species is completely dependent upon androgens for size maintenance and undergoes involution and programmed cell death following androgen withdrawal [460].
The impact of androgens on lacrimal gland secretion is also not the same in all species. Although androgens regulate the lacrimal gland output of certain proteins [13,98,100,102,103,105,377,412,413], these hormones do not elicit a consistent, species-independent action on fluid or total protein secretion [204]. Instead, androgens induce time-, strain- and species-dependent effects, leading to a non-uniform increase, decrease or no impact on the volume and total protein level of tears in mice, rats, guinea pigs, rabbits and humans [12,204,361,369,376,377,384,387,393,396,397,408,461–464]. Of particular interest is the observation that androgens upregulate the expression of cystatin-related proteins in the rat lacrimal gland [88,105]. Cystatin 4 (also called cystatin S), in turn, is one of the main discriminant protein biomarkers in the tear film for differentiating between people with and without DED [465,466].
3.1.1.2 Clinical relevance of androgen influence on the lacrimal gland
3.1.1.2.1 Sjögren syndrome
Androgen deficiency appears to be a risk factor for, but not a cause of, the development of lacrimal gland inflammation and aqueous-deficient DED in women with Sjögren syndrome. Women with Sjögren syndrome are androgen-deficient [391,467–472]. The serum levels of DHEA, 5-androstene-3β,17β-diol, DHT, androsterone-glucuronide (ADT-G) and androstane-3α,17β-diol -glucuronide (3α-diol-G) are significantly reduced in women with this autoimmune disorder [391]. The decrease in ADT-G and 3α-diol-G concentrations is noteworthy, because these glucuronidated DHT metabolites reflect the total intracrine production and metabolism of androgens in peripheral tissues and appear to be the most valid and reliable measures of the total androgen pool in humans [447,451,452].
An even greater androgen deficiency may exist in lacrimal tissues of Sjögren syndrome patients. The reason is that levels of proinflammatory cytokines, such as IL-1, TNF-α and IL-6 are elevated in exocrine tissues in Sjögren syndrome [85,373,430,473–479]. These cytokines may disrupt the normal activity of steroidogenic enzymes and promote the aromatization of testosterone to 17β-estradiol [480–484]. These cytokines may also attenuate the expression of androgen receptor mRNA [485], interfere with certain androgen actions [486] and stimulate corticosteroidogenesis, which potentiates the aromatization of androgens, resulting in decreased testosterone and increased estrogen levels [487]. This decrease in testosterone would enhance inflammation, given that androgens typically suppress the expression of TNF-α, IL-1β and IL-6 and potentiate the levels of the anti-inflammatory cytokine IL-10 [464,488–490]. In contrast, the increase in estrogen might also enhance inflammation, because this hormone may stimulate TNF-α, IL-1β and IL-6 production, synergize with IL-1β, and attenuate IL-10 amounts [488,491,492]. This androgen deficiency would compromise the positive regulatory influence of androgens in lacrimal tissues of Sjögren syndrome patients, and predispose to the development of glandular dysfunction, inflammation, reduced tear secretion and aqueous-deficient DED. Conversely, correcting this androgen deficit in Sjögren syndrome may have a therapeutic effect on the lacrimal gland.
In support of these deductions are the following observations. Testosterone treatment of female mouse models of Sjögren syndrome (i.e. MRL/Mp-lpr/lpr [MRL/lpr] and NZB/NZW F1 [F1]) causes a dramatic suppression of the inflammation in, and a significant increase in the function of, the lacrimal gland [348,358,374–377,384]. This hormone action may be duplicated by administering a number of “anabolic” or “androgenic” androgen analogues, but not by exposure to estradiol, danazol, cyclosporine A (CsA), dexamethasone or experimental non-androgenic steroids [13,374]. In addition, this anti-inflammatory effect seems to be site-specific: androgens decrease lymphocyte accumulation in lacrimal, as well as salivary, glands, but do not diminish the extent of inflammation in lymphatic and splenic tissues [376], or lessen certain systemic immune dyscrasias [493–495]. 19-nortestosterone administration to the ocular surface completely resolves the inflammation in nictitating lacrimal glands of dogs with DED [496,497]. Topical or systemic androgen administration significantly decreases DED signs and symptoms, and stimulates tear flow, in patients with Sjögren syndrome [394,396,397,461]. These findings support the hypothesis that androgen deficiency is a significant risk factor in the pathogenesis of lacrimal gland dysfunction, inflammation and aqueous-deficient DED in Sjögren syndrome.
That such an androgen-autoimmune interrelationship may exist is not surprising. Researchers have shown that androgen insufficiency may contribute not only to the prevalence of Sjögren syndrome, but also to the sex-specific expression of other autoimmune diseases. This sexual dichotomy, in turn, has been linked to the differential actions of sex steroid hormones on the immune system [487,498,499]. Estrogens often enhance, whereas androgens frequently attenuate, the progression of autoimmune sequelae [348,379,394,498–508]. In effect, androgen deficiency appears to promote the development of certain autoimmune states. This may explain why androgen therapy has been successful in alleviating various signs and symptoms in animal models of systemic lupus erythematosus, thyroiditis, polyarthritis, autoimmune hemolytic anemia, and myasthenia gravis, as well as in humans with rheumatoid arthritis and systemic lupus erythematosus [348,394,396,397,487,498,499,505–508].
The mechanism(s) by which androgens suppress lacrimal gland inflammation in Sjögren syndrome has yet to be clarified. One process, though, likely involves a hormone-induced decrease in inflammatory gene activity. For example, androgens suppress the lacrimal gland mRNA levels of caspase 1 [351], which is known to activate the pro-inflammatory cytokines IL-1β and IL-18, as well as promoting the efficient expression of IL-1α [509]. Androgens also reduce the lacrimal gland expression of MHC Class II antigen processing genes, as well as that for ASGPR 1, which has been implicated in the development of exocrine gland inflammation [207,208,211]. In autoimmune MRL/lpr mice, testosterone has been shown to significantly decrease the expression of genes related to inflammatory responses, immune cell chemotaxis and antigen presentation [510]. These androgen actions appear to be initiated through androgen binding to non-defective androgen receptors in lacrimal gland epithelial cells [372,511]. Acinar and/or ductal epithelial cells, in turn, are thought to be the primary cells involved in the initiation and perpetuation of autoimmune reactivity in Sjögren syndrome [512]. This androgen-epithelial cell interaction may then induce the altered activity of specific genes and proteins in lacrimal tissue (e.g. cytokines, proto-oncogenes and apoptotic factors), leading to the reduction of immunopathological lesions and an improvement in glandular function [348,358,374,375,377,384].
In contrast to the above, some investigators have proposed that androgen insufficiency causes an autoimmune process in the lacrimal gland, leading to inflammation, a Sjögren syndrome-like pathology and aqueous tear deficiency [361,362,513,514]. In support of this hypothesis, researchers have reported that androgen withdrawal triggers glandular atrophy, involving reduced acinar size, acinar cell necrosis and extensive regions of acinar cell degeneration [361,362,513]. These alterations are proposed to stimulate the generation of autoantigens, the development of lacrimal gland autoimmune disease and the induction of a Sjögren syndrome-like aqueous tear insufficiency [361,362,514]. Androgen administration, in turn, may prevent lacrimal gland regression [361,362,387], thereby suggesting that these hormones are essential for maintaining fluid secretion by lacrimal tissue.
However, these hypotheses are not supported by other studies. Lacrimal glands from young and old testicular feminized (Tfm) mice do not show any histological evidence of inflammation due to androgen deficiency [353]. These Tfm mice, which are often used to evaluate androgen-dependent phenomena, possess dysfunctional androgen receptors and are resistant to androgen influence [515]. Moreover, androgen synthesis in Tfm mice is severely reduced [516]. This insufficiency, when coupled with the androgen receptor defect, would serve to prevent both classical and nonclassical effects of androgens. Consequently, if androgen deficiency causes lacrimal gland inflammation, one might anticipate that this effect should be apparent in Tfm mice. Such inflammation, though, is not present [353]. Similarly, investigators have found that androgen deficiency caused by castration and/or interruption of the hypothalamic-pituitary axis does not induce any lymphocyte accumulation in, or regression of, the lacrimal glands in male and female rats, guinea pigs and/or rabbits [204,353]. Researchers have also demonstrated that androgen receptor dysfunction (e.g. Tfm mice) and androgen insufficiency (e.g. men taking anti-androgen medications) do not cause aqueous tear deficiency [353]. Lastly, complete androgen receptor absence induces premature ovarian failure [517,518], a condition in humans that causes a non-aqueous-deficient DED [519]. Overall, it appears that androgen deficiency promotes, but does not cause, the lacrimal gland inflammation in Sjögren syndrome. Further, androgen deficiency may impair lacrimal gland function, but does not seem to induce aqueous-deficient DED.
Lastly, it should be noted that androgens induce lymphocyte accumulation in the lacrimal glands of NOD mice [264,520]. These NOD mice have been proposed as models of Sjögren syndrome and develop lacrimal gland immunopathology [521–523]. However, in contrast to the situation in humans, as well as in MRL/lpr and F1 mice, it is the male, and not female, NOD mice that feature extensive autoimmune disease in their lacrimal gland [64,65,263]. This anomalous effect is mediated through the lacrimal microenvironment [64] and male-specific factors that cause CD4(+) CD25(+) Foxp3(+) regulatory T cell dysfunction [264], and contrasts with the androgen-induced suppression of inflammation in NOD salivary and pancreatic tissues [64,524,525].
3.1.1.2.2 Non-autoimmune disease
Researchers have reported that low serum concentrations of testosterone are also more prevalent in women with DED and correlate with the subjective severity of ocular symptoms [526]. However, the reason for this association is unclear, given that serum testosterone levels reflect only a very small fraction of the total androgen pool in women [446–458]. In fact, investigators have proposed that the measurement of serum testosterone in women may have little or no value except as an index of ovarian activity [447,449,451]. Intracrine synthesis in peripheral tissues, and not the ovary, is the primary source of androgens (or estrogens) in human females [447,449,451,453,455–457].
Researchers have also suggested that the decrease in serum androgen levels that occurs during menopause, pregnancy, lactation, or the use of estrogen-containing oral contraceptives may trigger the development of a non-immune type of DED, termed primary lacrimal gland deficiency. [387] Others have reported that ovariectomy, with [527] or without [528] Type II 5α-reductase inhibitor (i.e. finasteride) treatment, will cause aqueous tear deficiency – apparently due to a loss of androgens. If these suggestions and/or reports are correct, then they may represent a response that is sex-specific (i.e. females, not males). The reason is that extended (e.g. years) exposure to anti-androgen therapy for prostatic disease has no effect on the aqueous tear production (i.e. Schirmer test), as compared to that of age-matched, untreated controls [353]. Consequently, androgen deficiency in males who show no evidence of immune pathology (e.g. autoimmune disease) does not appear to promote the development of aqueous tear deficiency.
3.1.1.2.3 Secretory immunity
In experimental animals, androgens have been demonstrated to stimulate the lacrimal gland secretory immune system, which helps protect the anterior surface of the eye against microbial infection and toxic challenge [529–532]. This immune function is mediated primarily through secretory IgA (sIgA), which originates from plasma cells in the lacrimal gland [533,534], is transported across epithelial cells into tears by the polymeric Ig receptor (i.e. secretory component [SC]) [13] and acts to prevent viral invasion, inhibit bacterial colonization, interfere with parasitic infestation and suppress antigen-induced damage on the ocular surface [529–532]. In rats, androgens induce the synthesis and secretion of SC by lacrimal gland acinar epithelial cells, increase the concentration of IgA in lacrimal tissue and promote the transfer and accumulation of SC and IgA in tears [535]. Androgens also stimulate the lacrimal gland output of IgA, presumably through SC regulation, into tears of mice [377]. Considering that the promoter region of the human SC gene contains several putative androgen receptor binding sites [536], it may be that androgens also enhance SC production in the human lacrimal gland. Such an androgen-induced increase of SC synthesis was demonstrated in human lacrimal gland epithelial cells [537]. Thus, androgens may stimulate sIgA transport into human tears and thereby promote the maintenance of corneal and conjunctival integrity and the preservation of visual acuity. Further, androgen deficiency could possibly be a factor in the decreased levels of IgA in tears of DED patients [538], and especially those of patients with Sjögren syndrome [539].
3.1.2 Androgen regulation of the meibomian gland
3.1.2.1 Androgen influence and mechanism of action
The meibomian gland, a large sebaceous gland, is an androgen target organ. Androgens stimulate this tissue's function and appear to suppress its keratinization [2,10,462,540–544]. Androgen deficiency, in turn, is a risk factor for meibomian gland dysfunction and a corresponding evaporative DED [2,10,35,391,392,540–542].
Androgens are known to modulate the development, differentiation and lipogenesis of sebaceous glands [545–549]. Sebaceous gland activity and secretion, in turn, may be antagonized by orchiectomy or topical anti-androgen treatment [550–554]. Androgen actions on the meibomian gland appear to be mediated, at least in part, through binding to classical nuclear receptors. The meibomian glands of male and female rats, rabbits and humans contain androgen receptor mRNA and androgen receptor protein within acinar epithelial cell nuclei [426,427,543]. In addition, androgens regulate the expression of numerous genes in mouse, rabbit and human meibomian glands [547,555–560]. These effects appear to depend on the presence of functional androgen receptors [556,557]. However, whether all androgen effects on the meibomian gland are mediated through classical receptors remains to be clarified. It is possible that androgen action may also involve binding to membrane receptors, triggering of signal transduction cascades and associated changes in gene transcription [561,562].
Androgen activity in the human meibomian gland may occur primarily after local synthesis from adrenal precursors. Human meibomian glands contain the mRNAs for all the enzymatic machinery necessary for the intracrine synthesis and metabolism of androgens, including steroid sulfatase, 3β-HSD)-Δ-5Δ4-isomerase type 1,17β-HSD types 1 and 3, Types 1 and 2 5α-reductase, aromatase, glucuronosyl-transferase and sulfotransferase [427,459]. Further, 3α-HSD, 3β-HSD and 17β-HSD, at least, are translated in epithelial cells of the human meibomian gland [563].
Androgens exert a significant impact on gene expression in the human meibomian gland. For example, DHT regulates the expression of almost 3000 genes in immortalized human meibomian gland epithelial cells (IHMGECs) [560]. Most striking are the effects of DHT on lipid- and keratin-related genes. Androgens stimulate 25 different ontologies (with ≥5 genes) concerned with lipid biosynthesis, homeostasis, transport and binding, as well as with cholesterol, fatty acid, phospholipid and steroid dynamics [110]. This hormone response is similar to the androgen influence on mouse meibomian glands in vivo [90,547,558,559] wherein testosterone upregulates many genes linked to lipid metabolic pathways. DHT administration also led to a 40-fold decrease in the mRNA level of small proline-rich protein 2A (SPPR2A) in IHMGECs. The expression of this SPPR2A gene is significantly upregulated in human MGD [110], and it encodes a protein that promotes keratinization [564]. Keratinization, in turn, is a primary cause of MGD and so may contribute to tear film hyperosmolarity and evaporative DED [20]. The SPPR2A gene, as well as other genes related to keratinization, is also significantly downregulated by testosterone in meibomian glands of male and female mice [90,547]. These combined androgen effects, increasing lipogenesis and suppressing keratinization, may begin to explain how topical androgens increase the synthesis and secretion of meibomian gland lipids, prolong tear film breakup time and alleviate evaporative DED in humans (see below) [462,544]. In addition, these DHT effects on IHMGECs may account for why androgen deficiency (e.g. during anti-androgen treatment, complete androgen insensitivity syndrome [CAIS] and/or aging) is associated with altered meibum lipid profiles, a reduced quality of meibomian gland secretions, and keratinization of the meibomian gland ductal epithelium (i.e. orifice metaplasia) in humans [10,112,540–542].
Androgen treatment also causes a significant change in the expression of many other genes in IHMGECs, such as those associated with steroidogenesis, microbial protection, tissue development, oxidative stress, mTOR (mechanistic target of rapamycin) and PPAR (peroxisome proliferator-activated receptor) signaling, cell cycle, innate immunity and angiogenesis. DHT upregulates the mRNA levels of defensin β1, an antimicrobial peptide implicated in epithelial surface resistance to microbial colonization [565], as well as steroid-5α-reductase, α polypeptide 1, which catalyzes the conversion of testosterone into the more potent androgen, DHT [565]. This steroid effect appears to be a form of feed-forward control exerted by DHT on its own biosynthesis [566]. DHT increases the gene expression of leptin receptor, involved in the regulation of fat metabolism, glucose homeostasis, wound healing and the immune system [565], as well as FOXO1 (forkhead box protein O1), a transcription factor that mediates cell responses to oxidative stress [565] and is known to interact with androgen receptors [567], and stearoyl-CoA desaturase, an iron-containing enzyme that catalyzes the synthesis of unsaturated fatty acids. Similarly, testosterone increases stearoyl-CoA desaturase mRNA levels in male and female mouse meibomian glands [90,547], and the targeted disruption of this rate-limiting enzyme results in meibomian gland atrophy [568]. DHT also stimulates ontologies and pathways in IHMGECs related to peroxisomes, which are organelles involved in metabolism of fatty acids and other metabolites [565]. DHT stimulates PPAR, which may promote tissue differentiation [569,570] and mTOR, a serine/threonine protein kinase that may regulate cell growth, cell proliferation, cell motility, cell survival, protein synthesis and transcription [565,571,572], which is also activated by androgens in the prostate [573]. DHT downregulates genes in IHMGECs associated with cell cycle regulation, innate immunity and angiogenesis. Androgen exposure also suppresses the IHMGEC gene expression for matrix metallopeptidase 9, an enzyme elevated in the tear film in DED and known to promote corneal inflammation [574], as well as the lipopolysaccharide (LPS)-induced secretion of leukotriene B4 (LTB4), a pro-inflammatory mediator [575].
In animal studies testosterone has been shown to regulate the expression of over 1000 genes in the meibomian glands of male and female mice [90,547,555,557–559]. Many of the upregulated genes are associated with lipid metabolism (e.g. sterol regulatory element binding protein), lipid transport (e.g. lipocalin 3), sterol biosynthesis (e.g. 3-hydroxy-3-methylglutaryl CoA-reductase) and fatty acid metabolism (e.g. fatty acid synthase), as well as with intracellular protein transport, oxidoreductase activity, peroxisomes, mitochondria and early endosomes [90,547,555,557–559]. Testosterone also increases the activity of a series of genes that may be very important in the endocrine control of the meibomian gland [547]. Testosterone stimulates activity of 17β-HSD 7, an enzyme that catalyzes the interconversion of 17-ketosteroids with their corresponding 17β-hydroxysteroids [576]. This enzyme is critical for the metabolism of all active androgens and estrogens [576] and may mediate the local, intracrine synthesis of androgens from adrenal precursors in the meibomian gland. Testosterone stimulates insulin-like growth factor 1, a pleiotropic protein that stimulates sebocyte proliferation, differentiation and signaling [577], Estrogen receptor (ER) β, a receptor that is upregulated by androgen in the prostate [578] and may antagonize the activity of ERα [579] is stimulated by testosterone, as is 11β-HSD 1, an enzyme that converts cortisol to the inactive metabolite, cortisone.
Androgens also influence the lipid, and possibly protein, composition within the meibomian gland. Orchiectomy causes a marked alteration in the lipid profile of rabbit meibomian glands, whereas the topical or systemic administration of 19-nortestosterone, but not placebo compounds, for 2 weeks begins to restore the lipid pattern towards that found in intact animals [543]. In addition, investigators have speculated that androgen-signaling machinery regulates the expression of secretoglobin in human meibomian gland epithelial cells [580]. This protein may be released and possess a lipocalin-like function in the tear film [466,580].
3.1.2.2 Clinical relevance of androgen influence on the meibomian gland
Androgen deficiency is a risk factor for the development of MGD [2,20]. Such hormone insufficiency typically occurs during menopause (decrease in ovarian and adrenal ‘androgen’ secretion) [355,447,581], aging in both sexes (decline in the total androgen pool) [446,447], the use of anti-androgen medications (e.g. for prostatic hypertrophy or cancer) [582], CAIS (women with dysfunctional androgen receptors) [583,584], and autoimmune disease (e.g. Sjögren syndrome, systemic lupus erythematosus, rheumatoid arthritis) [391,430,585].
As an example, researchers have discovered that patients taking anti-androgen therapy, compared with controls, have significant alterations in their meibomian glands, including orifice metaplasia (i.e. a condition defined as an abnormal growth and keratinization of duct epithelium) [586], a decreased quality of secretions, a marked change in the neutral lipid profile of meibum, and a morphological appearance consistent with severe disease [540,541]. Many of the lipid alterations are “all” or “none,” the same in both eyes and feature characteristic shifts in fatty acid patterns [541]. Moreover, patients have a significantly greater frequency of tear film (i.e. debris, abnormal menisci, instability), conjunctival (i.e. tarsal injection, inferior staining), corneal (staining), and lid (i.e. irregular posterior lid margins, sleeves, collarettes) abnormalities, as well as increased ocular surface symptoms (i.e. light sensitivity, painful eyes, blurred vision) [540]. These observations extend those of another report, which linked leuprolide acetate treatment (i.e. to reduce testosterone levels) with ophthalmic problems and blurred vision in some patients [587]. In addition, these results may explain why a significant association exists between the use of medications to treat benign prostatic hyperplasia and DED [35].
Investigators have also discovered that androgen receptor dysfunction in CAIS individuals is associated with a significant increase in DED signs and symptoms [542]. These include a significantly reduced tear meniscus, a significantly increased degree of lid erythema, telangiectasia and keratinization, a significantly higher frequency of meibomian gland orifice metaplasia and irregular posterior lid margins, as well as a reduced meibum quality. These CAIS individuals also have striking changes in the neutral and polar lipid patterns of their meibomian gland secretions, compared to those of normal male and female controls [10]. In addition, aging in men and women is associated with a significant decrease in the quality of meibum and a significant increase in the frequency of metaplasia of meibomian gland orifices [112,588]. Aging is also accompanied by marked alterations in the polar and neutral lipid profiles of meibomian gland secretions [112,588]. These observations were made by comparing 37 and 70 year-old people, and this time period between the 4th and 8th decades coincides with a dramatic decline in androgen levels in both sexes [447]. For comparison, sebaceous gland function decreases with age [589]. This age-associated dysfunction has been correlated with both an atrophy of acinar epithelial cells and a reduction in serum androgen levels [589]. In fact, the age-related cellular shrinkage in certain sebaceous glands has been directly correlated with attenuation in androgen levels in the surrounding skin [589].
Additional studies have linked androgen deficiency with MGD and evaporative DED. Researchers have found that non-autoimmune DED patients with MGD are androgen-deficient [590], and others have discovered that the topical application of DHEA to a human, as well as to rabbits and dogs, stimulates the elaboration and release of meibomian gland lipids and prolongs the tear film breakup time [544]. Furthermore, investigators have reported that decreased serum concentrations of testosterone are more prevalent in women with DED and correlate with the subjective severity of ocular symptoms [526], and that serum testosterone concentrations correlate positively with meibomian gland secretion volume and orifice diameter in pre- and post-menopausal women, respectively [591,592]. This latter correlation is of interest, given that serum testosterone levels represent < 0.2% of the total androgen pool in women [450].
In summary, these findings indicate that the meibomian gland is an androgen target organ, that androgens promote lipogenesis and suppress keratinization within this tissue, and that androgen deficiency may lead to MGD and evaporative DED. This interrelationship between androgen deficiency, meibomian gland dysfunction and evaporative DED may help to explain why topical or systemic androgen administration have been reported to alleviate the DED signs and symptoms in women and men [394,396,397,410,411,461,462,544]. Consistent with these data are Phase 2 clinical trial results, which show that treatment of MGD with topical testosterone improves the quality of meibomian gland secretions and reduces ocular discomfort [593].
3.1.3 Androgen regulation of the cornea and conjunctiva
3.1.3.1 Androgen influence and mechanism of action
Androgens have been reported to stimulate the proliferation, dynamics and/or immune response of the cornea and conjunctiva [155,594–599]. Conversely, androgen deficiency has been linked to the development of corneal and conjunctival epitheliopathies [369,393].
The mechanism of androgen action in the cornea and conjunctiva appears to involve the local, intracrine synthesis of androgens from adrenal precursors (e.g. DHEA), binding to saturable, high-affinity and androgen-specific receptors, control of gene transcription, and ultimately modulation of translation. Human corneal and conjunctival epithelial cells contain all the steroidogenic enzyme mRNAs necessary for the intracrine synthesis and metabolism of androgens [427,459,600,601]. In addition, androgen receptor mRNA and protein are present in epithelial cell nuclei of the cornea and conjunctiva [389,427,602–605], and DHT is known to modulate the expression of almost 1500 genes in primary human corneal epithelial cells [606], as well as over 3000 genes in immortalized human conjunctival epithelial cells [560].
In primary human corneal epithelial cells DHT upregulates genes encoding syntenin (e.g. regulates transmembrane-receptor trafficking, tumor-cell metastasis, neuronal-synapse function and NF-kappaB activation pathways), decorin (e.g. inhibits angiogenesis) and vimentin (e.g. helps maintain cell integrity) and suppresses genes producing tubulins (e.g. promote angiogenesis) and matrix metallopeptidase 9 (e.g. degrades collagens) [606]. DHT treatment also exerts a significant impact on many ontologies, including oxidative phosphorylation, transmembrane ion transport and cellular localization [606]. In contrast, DHT treatment of immortalized human conjunctival epithelial cells enhances the expression of genes involved in epithelial development, regeneration, wound healing and cell migration (e.g. matrix metallopeptidase), and suppresses those related to the immune response (e.g. chemokine (C-X-C motif) ligand 6) and mitotic cell cycle (e.g. septin 4, endothelin 1) [560].
3.1.3.2 Clinical relevance of androgen influence on the cornea and conjunctiva
Androgen treatment has been demonstrated to stimulate mitosis, repair defects and facilitate wound healing, and to suppress angiogenesis and dystrophies in the cornea [155,594–598]. Moreover, androgens have been shown to alter the progression of allergic conjunctivitis [599] and to suppress the LPS-induced secretion of LTB4 in both human corneal and conjunctival epithelial cells [575].
In contrast, androgen deficiency is associated with corneal and conjunctival damage. Patients on anti-androgen therapy have a significant decrease in their tear film breakup time (TBUT), as well as a significant increase in the degrees of corneal fluorescein and rose bengal staining and inferior bulbar conjunctival rose bengal staining [540]. In addition, CAIS individuals have increased lid erythema, telangiectasia and keratinization [542], a greater frequency of irregular posterior margins, conjunctival erythema, and bulbar and tarsal injection [542], and a decreased expression of MUC1 [607]. This impact of androgen deficiency on the ocular surface has also been observed in animals. Investigators discovered reduced TBUT, increased corneal fluorescein staining, shorter and flattened corneal epithelial microvilli, and loose intercellular desmosomes in mice after orchiectomy [393]. These alterations were largely resolved following DHT treatment [393]. For comparison, complete androgen receptor absence in mice leads to premature ovarian failure, a condition in humans that is linked to heightened ocular surface damage, characterized elevated corneal fluorescein staining, increased conjunctival lissamine green staining, and DED symptoms [519]. Conjunctival, but not corneal, changes were also found in rabbits following orchiectomy, including increased rose bengal staining and decreased numbers of goblet cells [369].
Androgens have also been linked to the development of keratoconus in mice [443], and hyperandrogenism to an “itchy-dry eye” in women with polycystic ovary syndrome [608,609]. The polycystic ovary syndrome patients had a reduced TBUT, conjunctival hyperemia and increased goblet cell density [608]. However, the extent to which the ocular findings in polycystic ovary syndrome relate to excess androgen levels is unclear. Polycystic ovary syndrome patients also have insulin resistance, which leads to Type II diabetes, which in turn leads to DED [610–612].
3.1.4 Androgen role in sex-related differences of the ocular surface and adnexa
Androgen influence seems to account for many of the sex-related differences in the anatomy, molecular biology, physiology, immunology and disease susceptibility of the lacrimal gland in a variety of species, including mice, rats, hamsters, guinea pigs, rabbits and humans [12,14,82,87,100,102,103,105,195,199,202,205,249,351,379,390,395,400–405,407,409,412,417,613–617]. For example, of the hundreds of genes more highly expressed in lacrimal glands of male, as compared to female, mice, greater than 80% of those genes are also up-regulated by testosterone [351]. Similarly, of the hundreds of genes that are expressed significantly less in glands of male, relative to female, mice, more than 65% of those genes were also down-regulated by testosterone. Further, the top 10 to 14 biological process, molecular function and cellular component ontologies regulated by testosterone in mouse lacrimal glands are identical to those found to be influenced by sex [83,351]. In contrast, estrogen (15%) and progesterone (2%) influence only a small percentage of those lacrimal gland genes [352] reported to vary significantly between male and female mice [83].
Androgens are also known to exert sex-specific actions in the lacrimal gland [90]. Researchers have reported that sex steroid actions on the transcription of certain genes, the translation of the corresponding proteins, and the development of paradoxical inflammation in the lacrimal gland are sex-biased [11,64,86,90,105,480,618]. Such sex specificity of hormone action is not unusual. Sex steroids induce sex-specific effects in many cells (e.g. neutrophils, antigen-presenting cells and preadipocytes) and tissues (e.g. muscle, liver, hippocampus, spinal cord and vasculature) [618–625]. Sex steroids may also elicit opposite [626–630], and even antagonistic [631], influences, such as on sebaceous gland activity. Androgens and estrogens may often induce opposite responses in the lacrimal gland [90]. These responses may contribute to estrogen's possible pro-inflammatory [376], and androgen's anti-inflammatory [358,374,376,384], effects in lacrimal tissue in Sjögren syndrome.
The differential action of sex steroids, as in other sebaceous glands [548,552,632–634], may also play a role in the sexual dimorphism in the morphological appearance, gene expression, neutral and polar lipid profiles, and secretory output of the meibomian gland [6–8,18,19,35,112,113,188,543,588,635]. Androgens mediate almost 30% of the sex-associated differences in gene expression of the mouse meibomian gland [8]. In addition, androgens may contribute to the significant sex-related differences found in the lipid profiles of human meibomian gland secretions [112]. Analogous sex-associated differences exist in the mass/charge ratios of some neutral, but especially polar lipids in human meibum, and these have been linked to the presence of functional androgen receptors [543].
As in the lacrimal gland, androgens also have sex-specific effects in the meibomian gland. For example, several genes are up- or down-regulated by testosterone in the female, but not male, mouse meibomian gland [90]. In addition, a number of the androgen-regulated genes in female glands are altered in the opposite direction by 17β-estradiol and/or progesterone [90]. These genes could be involved in cell maturation, migration and holocrine secretion in the meibomian gland. If so, this would be consistent with a pro-sebaceous action of androgens and an anti-sebaceous effect of estrogens.
In the human corneal epithelium, males, as compared to females, have a significantly higher expression of genes associated with DNA replication and cell migration [9]. These sex-associated differences may be due to the influence of androgens, given that these hormones are known to stimulate mitosis in the corneal epithelium [155].
3.2 Estrogen and progesterone regulation of the ocular surface and adnexa
In contrast to androgen, the role of estrogen at the ocular surface is less well defined, with effects that appear to be tissue-, sex-, and dose-specific.
3.2.1 Estrogen and progesterone presence at the ocular surface
Analysis of intra-tissue estrogen or progesterone levels in ocular tissue has not as yet been undertaken. Both estrogen and progesterone have been detected in human tears, and are reported to be correlated with levels in serum of premenopausal females [636]. A study of progesterone and DHEA found tear levels to be substantially lower than those in serum, with no difference between males and females [637]. Other than these two conference abstracts, no investigation of sex hormones in tears has been published, reflecting the difficulties in detecting low concentration compounds, such as sex hormones, in small volumes of tears.
As is the case for testosterone, there is substantial evidence for biosynthesis and metabolism of estrogen at the ocular surface, which implies that it exerts a biological influence here. Aromatase, steroid sulfatase and hydroxysteroid dehydrogenases (17β HSD-1 and 3; 3α and β HSD) mRNAs have been identified in human meibomian and lacrimal gland tissue and in immortalized corneal and conjunctival epithelial cell lines [459]. If these mRNAs are translated, this means that human ocular surface tissues possess the enzymes necessary for the conversion of the precursor DHEA-S into estrogen in the process of intracrinology (Fig. 1). Intracellular synthesis and metabolism of sex hormones is a process unique to primates, and as such suggests caution for extrapolation of rodent studies with estrogens to humans.
Intracrine sources account for all estrogen synthesis in postmenopausal women, the majority of estrogen in men and up to 75% of estrogen synthesis in women prior to menopause [449,638]. In women after menopause, intracrine metabolism is an evolutionary measure which limits exposure of other tissues (specifically the endometrium) to estrogen, while providing estrogen to tissues where it continues to have an important function throughout life, including bone, brain, vascular tissue and presumably the ocular surface. Hence, in postmenopausal women and in men, local rather than systemic estrogen levels would be expected to direct estrogen action in peripheral tissue, including the ocular surface [639]. Circulating estrogen levels in postmenopausal women are the sum of small amounts of ‘leakage’ from various peripheral tissue sources, and as such, are not a good measure of estrogen influence at the ocular surface. Serum estrogen levels may be more relevant to the ocular surface of women prior to menopause.
Intracrine synthesis of estrogen is dependent on circulating levels of precursors, specifically DHEA, but also testosterone. Levels of serum testosterone are an order of magnitude greater than circulating estrogen levels in postmenopausal women and thus probably an important source of estrogen in peripheral tissues [639]. Thus, further to its direct androgen action discussed in the section above, testosterone has an important influence on estrogen action through its aromatization to estrogen in target tissues, including the ocular surface. In men, serum levels of testosterone are another order of magnitude higher than those in postmenopausal women [639], and thus may be an even more important source of estrogen at the ocular surface. Progesterone, in contrast, is primarily formed from cholesterol within the adrenal glands and the ovaries, and its intracrine synthesis is not well described.
3.2.2 Estrogen and progesterone receptors at the ocular surface
The presence of estrogen and progesterone receptor mRNA and receptor proteins is further evidence that ocular surface tissues are target sites for these hormones. In humans, mRNA for estrogen receptors (ER) has been identified in meibomian glands [426], lacrimal gland [426,640], cornea [426], bulbar conjunctiva [426,640,641], and in the tarsal plate [640]. It has been confirmed that these mRNAs are translated into proteins. ERs are present in the human meibomian gland, with an age-related increase in number of cells expressing ERs and no sex-related differences in distribution [642,643] (Fig. 2). A similar increase with age has been reported for estrogen and progesterone receptors (PR) in lacrimal gland cells, where they may be more frequent in women [644] (Fig. 3). ER and PR have also been identified in corneal epithelial, stromal and endothelial cells [604] and in bulbar conjunctival epithelium [641]. Conjunctival cells expressing ER or PR have not yet been investigated in men or in postmenopausal women. In human meibomian gland and corneal cells, the majority of ER appear to be the ERα type [604,643], whereas both ERα and ERβ are expressed in conjunctival epithelium [641]. In other tissues, ERα and ERβ mediate different functions of estrogens and thus a more complete characterization of the relative distribution of these receptor types at the ocular surface may provide important clues to understanding the role of estrogens in dry eye. Membrane receptors for sex hormones have been identified in human corneal and meibomian gland epithelial cells, and these may also play a role [645].
Fig. 2 Cross-section through normal human meibomian glands in the upper eyelid. Immunohistochemical staining shows nuclear positivity for estrogen receptors in basal and parabasal cells of acini (arrow).642 Reprinted from Ophthalmology 107, Esmaeli B, Harvey JT, Hewlett B, Immunohistochemical Evidence for Estrogen Receptors in Meibomian Glands, Pages No. 180–184, Copyright 2000, with permission from Elsevier.
Fig. 3 Estrogen (A) and progesterone (B) receptors in lacrimal gland acinar cells. Variable intensity of expression [644]. Reprinted from Archives Biological Science 63, Gligorijevi? J, Krsti? M, Babi? G, Immunohistochemical detection of estrogen and progesterone receptors in the human lacrimal gland, Pages No. 319–324, Open-Access article distributed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
3.2.3 Estrogen and progesterone mechanism of action at the ocular surface
3.2.3.1 Classic receptor action – regulation of gene expression
Estrogen and progesterone appear to act directly on their respective receptors to modulate the expression of genes that alter biological processes, molecular functions and cellular components in the lacrimal gland, the meibomian gland and in corneal epithelium in mice [117,352,646]. However, studies to determine whether estrogen and progesterone act through such classical receptors in human ocular surface tissue are yet to be carried out.
3.2.3.2 Non-genomic action
Estrogen and progesterone may also act on ocular surface tissue in an indirect fashion, through non-nuclear signaling pathways or via control of other pituitary hormones [647]. Non-nuclear signaling systems are important when immediate responses are required to maintain homeostasis of the tear film and ocular surface. Whereas the classic genomic mechanisms take hours or days to effect action, non-genomic signaling pathways at the membrane or in the cytoplasm can result in responses that occur much more rapidly, on the order of seconds or minutes [648–650]. Membrane bound estrogen receptors have been identified in human cells in non-ocular tissues [648,649], and receptors distinct from ERα, ERβ and PR or their isoforms may also be involved [650–653]. The involvement of alternate signaling pathways by estrogen in ocular tissue is supported by a study which showed that estradiol downregulates cyclic signaling in human meibomian gland epithelial cells [654].
3.2.3.3 Immunity and inflammation
Estrogen has well-known effects on the immune system. These actions are often dose-and tissue-dependent and may be completely different depending upon on the estrogen concentration. These differential effects may underlie some of the contradictory results found in studies evaluating estrogen for the treatment of dry eye disease [655,656].
Many types of immune cells, including T and B lymphocytes, dendritic cells, macrophages, neutrophils and natural killer cells express receptors for estrogen, progesterone and testosterone and hence can respond to these hormones [225,226,657]. In general estrogen enhances immune responses promoting production of B cells and antibody, sub-sets of T cells, dendritic cells, M2 macrophages and regulatory cytokines, while progesterone and androgens tend to decrease immunity [34,658]. Estrogens are the most widely studied of the sex hormones in terms of effects on immunity [225,237,245].
For example, estrogens inhibit the differentiation of Th17 cells in mice likely by down regulation of transcription factors including RORγT [659,660]. This is an interesting observation given the role of Th17 cells in the pathogenesis of dry eye [661] and greater prevalence of the condition in post-menopausal women. Notably estrogen, acting via ERα, inhibits Th1 and Th17 differentiation and is protective in a model of experimental autoimmune encephalomyelitis [662].
3.2.4 Estrogen and progesterone influence on lacrimal gland structure and function
Estrogen and progesterone affect the anatomy and physiology of the lacrimal gland, although there is conflicting evidence regarding the nature and extent of this influence. A gene array study of lacrimal glands in mice identified hundreds of genes that are regulated by 17βestradiol and progesterone [352]. The effects of 17βestradiol, progesterone, and 17βestradiol plus progesterone on gene expression were found to be mostly unique to each specific treatment. A study of aromatase knockout mice showed that the absence of estrogen also impacts the expression of numerous genes in the lacrimal gland [89].
Genes influenced by estrogen and/or progesterone treatment in mice included genes involved in transcriptional regulation, cell growth, division and/or maintenance, cell communication, signal transduction, enzyme catalysis, nucleic acid, protein and lipid metabolism, neurogenesis and a diverse array of immune-related genes [352]. Genes stimulated by 17βestradiol included actions in signaling pathways, ion transport, enzyme activities, and membrane aspects, whereas genes suppressed by estrogen were involved in cell organization and biogenesis, cytokine activity, receptor binding, mitochondria, and intracellular components [352]. Administration of progesterone increased expression of genes linked to signal transduction and cell communication and attenuated expression of genes linked to cell growth, maintenance, metabolism, and ion binding [352]. Interestingly, combined 17βestradiol and progesterone treatment increased expression of cell death genes and decreased that of genes related to receptor binding, signal transduction, protein transport, cytokine activity, and development [352]. 17βestradiol and progesterone were found to only modulate a small proportion of genes regulated by androgens, however, these effects were often in a direction opposite to that of androgen [663]. Although caution is urged in applying findings from mice to humans [664,665], these results are supportive of an important role of estrogen and progesterone in the lacrimal gland, which is beyond that of modulation of inflammation. In addition, these gene data represent mRNA levels, and translation into proteins is yet to be determined. However, if translation does occur, and if even some of the findings in mice are transferrable to humans, such alterations in gene activity influenced by estrogen and/or progesterone may be associated with ocular surface effects which lead to dry eye.
It has been proposed that estrogen and progesterone may have an anti-inflammatory influence in the lacrimal gland, and estrogen deficiency from ovariectomy or anti-androgen treatment in animals has been reported to negatively impact the lacrimal gland. In rabbit and mouse models of Sjögren syndrome, absence of estrogenic influence is reported to lead to regressive, inflammatory changes in lacrimal gland tissue, while estrogen administration prevents and/or reverses these changes and promotes lacrimal secretion [513,666,667]. The finding that estrogen with or without progesterone treatment influences the expression pro-inflammatory genes in the mouse lacrimal gland may support this thesis [352]. However, a more recent study of aromatase knockout (ArKO) mice, demonstrated that although estrogen absence exerted a substantial impact on expression of thousands of genes in the lacrimal gland, it did not significantly induce upregulation of genes related to inflammatory pathways [89]. In the same study, no histological evidence of lacrimal gland inflammation was observed, and tear volume was not reduced in ArKO mice. This lack of effect on tear volume is different than found following aromatase inhibitor treatment of postmenopausal women in which development of dry eye was reported [668–670]. It may be that increased circulating levels of androgens and other hormones in the sera of ArKO mice contribute to the difference found with the human studies [89].
In contrast, a number of studies in humans and animals show that estrogen and/or progesterone have a negative influence on the lacrimal gland. Estrogen action on the lacrimal gland promotes inflammation and autoimmune disease in some conditions [376]. Estrogen treatment of rabbits and mice increased the activity, level and expression of MMP-2 and -9 in the lacrimal gland [464,671]. This effect was not apparent in mice treated topically [464]. Other studies found no effect of estrogen treatment on lacrimal gland structure or protein secretion in humans or rats [12,14,412,672,673].
The findings from numerous investigations, in rodents and in humans, of the influence of estrogen on the structure and function of the lacrimal gland are summarized in Table 6 [19,60,89,350,352,355,464,513,519,647,666,668–671,674–683]. Contradictions apparent in these findings may be explained to some degree by differences in study design, treatment dosage, patient cohort or animal models (including sex-difference, Section 2.4). Conflicting results may also be due to the influence of other hormones (see Sections 3.1., 3.3–3.6) and as yet unidentified pathways that influence lacrimal gland function.
3.2.5 Estrogen and progesterone influence on meibomian gland structure and function
The nature of estrogen's and progesterone's influence on meibomian gland function is yet to be completely understood. Gene array studies in mice suggest that estrogen and progesterone have multiple effects on meibomian gland function and may possibly decrease lipid synthesis. The evidence in humans from population and clinical studies may support this contention (see Section 3.2.7). Estrogen and progesterone have also been reported to alter the morphology of mouse meibomian glands, involving changes in the area and/or number of acinar epithelial cells [6].
The meibomian gland is a large sebaceous gland, and there is substantial evidence supporting the negative impact of estrogen on the structure and function of sebaceous glands in other tissues and in various species [20,213,631,632,684–686]. The use of estrogen or combined estrogen and progesterone treatment to reduce sebum production in acne vulgaris is a well-known example [687,688]. Of note, the impact of estrogen and progesterone in sebaceous glands is tissue-, sex- and species-specific [213].
In the meibomian gland, estrogen and progesterone modulate expression of genes that influence biological processes, molecular function and cellular components in mice [117,646]. Importantly, estrogen opposes some actions of androgen in the meibomian gland. Estrogen suppresses genes associated with support of lipid production and upregulates genes which have the opposite effects [646,663]. In mice treated with 17βestradiol, progesterone or a combination, a gene microarray study showed that both hormones modified expression of almost 200 genes in the meibomian gland [646]. Overall, estrogen might have a negative influence on meibomian gland lipid generation and secretion due to its downregulation of genes involved in lipid biosynthesis, acinar cell maturation, migration and holocrine secretion, and its upregulation of genes involved in lipid and fatty acid metabolism [646]. In addition, estrogen was shown to modulate, albeit in smaller numbers, genes associated with promotion of lipid production, immune factors, tyrosine kinases and steroidogenesis [646]. Whereas 17βestradiol upregulated and downregulated equal numbers of genes, the impact of progesterone treatment in the mouse meibomian gland was of smaller magnitude and mostly via downregulation of gene expression [646]. Progesterone's most prominent influence was in suppression of genes regulating protein, macromolecule biosynthesis and ribosome biogenesis. Combined 17βestradiol/progesterone treatment mostly mirrored the effects of either hormone administered alone, but also influenced additional gene ontologies, and in some cases had opposing effects on gene expression [646]. Overall, these various steroid treatments led to many analogous, opposite, or unique effects on gene expression [646].
A subsequent gene microarray study of wild type and ArKO mice showed that aromatase (and by extension estrogen) presence and absence significantly influenced the expression of over 1000 genes in the meibomian gland, and that the nature of these effects was highly sex-dependent [117]. Of note, aromatase deficiency in female mice resulted in significantly increased expression of genes encoding a variety of immune functions, whereas mitotic ontologies were increased in male mice [117]. The sex-related variations were similar in ArKO and WT mice, indicating that aromatase and estrogen do not play a primary role in the sex-related differences in the meibomian gland [117]. Interestingly, the majority of genes significantly influenced by estrogen absence in the meibomian gland were different to those identified in the lacrimal glands [89], which is further evidence for the tissue-specific nature of the impact of estrogen on the ocular surface.
Aromatase (and thus estrogen) deficiency did not have an effect on the histology of meibomian glands in male of female mice, and no signs of inflammation were evident [117]. In contrast, an earlier study reported changes in meibomian gland morphology, involving changes in the area and/or number of acinar epithelial cells, in mice treated with estrogen [6]. The difference may relate to the increased serum levels of androgens and other hormones in the ArKO mice [89].
As in the lacrimal gland, results in mice may not be directly transferable to humans [664,665], and the findings discussed require confirmation in human trials. Evidence of estrogen suppression of cAMP signaling in, and the proliferation of, immortalized human meibomian gland epithelial cells supports the gene array findings in mice [654]. Furthermore, a number of clinical studies report relationships between higher estrogen levels and reduced tear stability and meibomian gland secretion [313,355,689]. In contrast however, improved tear stability has been reported in studies of women undergoing hormone replacement therapy (HRT) and topical estrogen administration (Table 7) and these are discussed below (see Section 3.2.7).
3.2.6 Estrogen influence on corneal and conjunctival structure and function
In addition to the lacrimal and meibomian glands, it is likely that estrogen has a direct influence on the epithelial cells of the cornea and conjunctiva. Although the weight of the evidence is on estrogen's impact on conjunctival tissue, the inherent difficulty of obtaining corneal cells from living humans has limited the in vivo studies examining the effect of estrogen on the human cornea.
3.2.6.1 Cornea
In human corneal epithelial cell culture, estrogen treatment of primary cells results in reduction of IL-6 and IL-8 mRNA levels and has no effect on IL-1β, matrix metalloproteinase (MMP) -2 or MMP-9 gene expression [690]. In contrast, in immortalized (SV40) cells, estrogen treatment has been shown to upregulate expression of a variety of proinflammatory cytokine genes (IL-1β, IL-6, IL-8, GM-CSF) and MMP-2, -7 and -9 genes [690,691]. The increased expression of MMP-2 and MMP-9 mRNA was not accompanied by an increase in protein translation and consequent secretion. Interestingly in another study, when SV40-immortalised corneal epithelial cells were exposed to a hyperosmolar medium (450 mOsm/L) simulating the conditions of dry eye, pre-treatment of cells with estrogen inhibited the mRNA expression and production of the proinflammatory cytokines IL-6, IL-1, TNF-α [692]. Another study of SV40-immortalised human corneal epithelial cells showed faster rates of cell migration in response to estrogen, as well as higher (dose-dependent) levels of epidermal growth factor and increased gene expression of fibronectin [693]. This suggests estrogen could have a beneficial effect on wound healing, although these findings are yet to be replicated in primary cells. The SV40-immortalised cell line does not represent normal corneal cells with respect to the estrogen regulation of cytokine and MMP responses, perhaps as a result of differences in ERα and ERβ expression [690].
Changes in corneal sensitivity have been reported in relation to altered estrogen levels during the menstrual cycle, albeit these are also conflicting. Reduced corneal sensitivity has been reported to occur at the estrogen peak in the ovulatory phase [220] and also during the menstrual phase during which lowest estrogen levels are reported [694]. No alterations in corneal sensitivity were detected following transdermal estrogen treatment in a group of post-menopausal women with dry eye [689].
3.2.6.2 Conjunctiva
Utilizing conjunctival smears processed with the Papanicolau technique (“Pap smear”), epithelial cell morphology was examined in menstruating and postmenopausal women [695] and also following HRT [696]. Cyclical changes were demonstrated in the conjunctival cells which were similar to (albeit milder than) those seen in the cells of vaginal epithelium, with a shift towards more mature (i.e. intermediate/superficial) cells during the estrogen peak at ovulation. During the menstruation phase with lowest circulating estrogen levels, parabasal cells dominated, as they did in the conjunctival smears of the postmenopausal group, which also reflected the pattern seen in vaginal cells. A subsequent study demonstrated an increased proportion of mature cells in postmenopausal women following transdermal estrogen and progesterone treatment [696]. Similar cyclical variations with menstrual cycle were reported in a later study [313]. Increased conjunctival goblet cell density has also been reported following HRT [697]. A recent report did not find sensitivity changes following transdermal estrogen treatment in a group of post-menopausal women with dry eye [689].
3.2.7 Clinical relevance of estrogen and progesterone action in DED
The clinical evidence regarding the influence of estrogen and progesterone on dry eye is inconsistent, with epidemiological evidence suggesting a negative impact with exogenous hormone administration in women, whereas smaller intervention studies show a range of effects following treatment, which may be related to dose and menopause status or ovarian function. Observational studies suggest circulating estrogen levels may have differing effects in pre and post-menopausal women. Apart from epidemiological investigations, studies of estrogen effects on dry eye have been carried out exclusively in women.
Although a common assumption exists in the literature that menopause is associated with increased occurrence of dry eye, a closer inspection shows that definitive evidence is lacking. As discussed above (Section 2.2), the prevalence of dry eye is higher in females than males across the lifecycle and dry eye prevalence increases gradually with age in both men and women [18,35]. Such a gradual increase in both sexes is perhaps more in keeping with the gradual decrease in serum androgen levels which occurs with age rather than the abrupt decline in ovarian estrogen at menopause. However, more dry eye symptoms were demonstrated in a study of 17–43 year old women with premature ovarian failure, than in an age-matched control group [519], alluding to a positive role for ovarian estrogen. Three reports of increased risk of dry eye in postmenopausal women undergoing aromatase inhibitor treatment for breast cancer highlight the importance of peripheral estrogen synthesis in ocular surface homeostasis [668–670].
Two observational studies of postmenopausal women have shown higher endogenous serum estrogen levels to be associated with increased osmolarity, reduced tear secretion (Schirmer I) and stability, and with meibomian gland dysfunction [355,689]. In accord with these findings is reports of reduced tear stability (measured using Schirmer I) [466] and higher maturation of conjunctival epithelial cells during the ovulation phase of the menstrual cycle, when serum estrogen levels peak [313]. However, reduced tear stability was not confirmed in another study [312], and neither study showed changes in tear secretion (Schirmer I). Both studies reported higher symptoms of dry eye during the estrogen peak at ovulation and during the late luteal phase when levels of progesterone are also high. Alterations in corneal sensitivity during the menstrual cycle have also been reported [220,694]. Other investigations have not found variation of ocular discomfort or of tear function measures including tear secretion, stability, osmolarity and evaporation, over the course of a menstrual cycle [683,698–701].
A large population-based study of 25,665 postmenopausal women from across the U.S. showed higher risk of dry eye for women using hormone replacement therapy (HRT), particularly with estrogen-only therapy [60]. HRT use was also identified as a risk factor in a smaller Australian study [19]. These epidemiological findings are supported by smaller clinical studies showing systemic estrogen and progesterone administration during HRT to have a negative effect on symptoms [689,702], tear secretion [674] and dry eye signs [703] (Table 7). Other clinical evidence, however, suggests estrogen supplementation with or without progesterone improves dry eye symptoms [675,679–681,704,705], tear secretion [675–681,706] and tear stability [676,677,681,697,706], and may have positive effects on the conjunctiva [696,697] and possibly on the meibomian glands [707] (Table 7). Importantly, however, none of the studies reporting improvement in symptoms were placebo controlled. Of the three placebo-controlled studies, one showed a worsening of symptoms with treatment [689] and two reported no change [676,708]. Also important to note is that studies of topical application of estrogen to the ocular surface or ocular adnexa showed improvement in tear function, although the estrogen doses used in both studies were 2500 and 300,000 times the normal physiologic levels of estrogen [675,676,709]. As an additional consideration, all patients in the Sator study were treated with systemic estrogen and progesterone [675]. The topical placebo, but not topical estrogen, group was given benzalkonium chloride-containing drops. It is not clear to what extent the results reflect a toxic effect of benzalkonium chloride, as compared to a positive effect of estrogen.
Estrogen supplementation in women pre-menopause is also not conclusive. Anecdotal evidence and case reports imply that oral contraceptive use may be associated with increased symptoms of dry eye and contact lens intolerance. However these observations have not been confirmed by formal investigations, which found that oral contraceptive use did not impact ocular symptoms, tear osmolarity, tear secretion, tear stability or other aspects of tear physiology [698,699,710]. Of possible relevance is a case series of two instances of dry eye and reduced tear secretion in young women, associated with development of Sjögren syndrome following high dose estrogen treatment [711].
Some of these contradictions may be explained by estrogen inducing differential actions over a range of concentrations and on different tissues of the ocular surface. Estrogen has been shown to have differential effects on inflammation in various tissues at differing circulating levels (see Straub [656] and Lang [655] for review), although this has yet to be shown in the eye. The effects of estrogen on the ocular surface need to be considered in context of the concurrent actions of other hormones, including progesterone and in particular the androgens. Combination estrogen/progesterone treatment has differential effects on gene expression in lacrimal and meibomian glands compared to estrogen alone (discussed above). Thus the relative ratios of the levels of these hormones should also be taken into account in clinical assessments. It may be the reduction in androgen action, rather than increased estrogen action per se, that is responsible for the higher prevalence of dry eye in women. Most importantly, due to the intracrine synthesis of estrogen particularly after menopause, assessment of local tissue levels of estrogen, rather than serum levels, is more appropriate and may yield more consistent clinical relationships with indicators of dry eye.
A more consistent approach to study design will allow more definite conclusions to be drawn. Of the treatment studies summarized in Table 7, only two are placebo controlled, an essential component when assessing a condition in which subjectively reported symptoms are a primary feature. Other clinical studies are underpowered to examine dry eye indices, including symptoms and tear function, which have large variability. The use of convenience samples of women undergoing HRT has made it difficult to control for treatment dose and duration or patient cohort. Importantly, few studies report measures of treatment compliance or bioavailability such as circulating or local hormone levels.
3.3 Glucocorticoid regulation of the ocular surface and adnexa
3.3.1 Glucocorticoids in ocular surface physiology and inflammation
Glucocorticoids (GC) are important endogenous regulators of the inflammatory response exerting potent anti-inflammatory effects in the body through the activation of the glucocorticoid receptor (GR), a nuclear hormone receptor that modulates gene transcription. The GR is a cytoplasmic protein, residing in an “aporeceptor” complex together with heat shock proteins 70 and 90, immunophilins, and p23 [715]. When the GR is associated with the GC ligand, the aporeceptor partially dissociates, enabling the GR to translocate to the nucleus, where it binds genomic GC-response elements (GRE) and regulates transcription of associated genes. Depending on the type of GRE activated, the activity of GC can be either activation or repression of gene transcription. The activity of the GR depends on the presence of cofactors (coactivators or corepressors), essential to conduct the signal to the transduction machinery and to chromatin, to obtain the necessary nucleosome remodeling and altered DNA topology. Among these cofactors, the most studied are the p160 proteins family, including the glucocorticoid receptor-interacting protein 1 responsible for the activation of the so called tethering GRE, which mediate the inhibitory effects of GC on transcription and, therefore, their immunosuppressive/immunomodulating activity [716–721].
As the primary physiological anti-inflammatory and immunosuppressive hormones in mammals, GC can interfere with multiple steps of both the innate and adaptive immune responses [722]. Also synthetic derivatives of these hormones are widely prescribed as anti-inflammatory agents including for ocular surface conditions. Topical GC are used as short-term therapy for the treatment of moderate [723] and severe dry eye disease [724–726] and related ocular surface inflammation. However, long-term use of these hormones may increase the risk for the development of infection, glaucoma and cataracts.
Under physiological conditions, endogenous GC may exert an autocrine effect in cells such as epithelial cells and fibroblasts, contributing to immunoprotection of the ocular surface mucosa through cortisol production [727]. Two different systems control cortisol production in the body: the hypothalamic-pituitary-adrenal axis for systemic production and the 11β-hydroxysteroid dehydrogenases (11β-HSDs) for local production. The latter is the most important system for the regulation of the inflammatory/anti-inflammatory activity taking place in peripheral tissues such as the ocular surface. Two main GC are produced in the cells: cortisone and cortisol, the latter being the active form. The local production of cortisol depends on the activity of enzymes, such as the 11β-HSDs, which regulate the production of cortisol from cortisone, a reaction dependent on NADPH as cofactor [728], and vice versa. Two 11β-HSD isoforms have been described: 11β-HSD1, which promotes the production of cortisol and 11β-HSD2, which converts cortisol to inactive cortisone [729,730]. The balance between the production of cortisone and cortisol is a key point in the control of the events leading to activation of innate and adaptive immune responses and 11β-HSD1 has been shown to play a fundamental role in helping balance monocyte maturation and immune-cell function with the control of tissue damage consequent to the inflammatory process [731,732]. At the ocular surface, 11β-HSD1 has been found localized to the basal cells of the central corneal epithelium while the serum and glucocorticoid regulated kinase 1 (SGK1), a target gene for the GR, was localized into proliferating limbal cells [716]. At the ocular surface, under physiological conditions, the autocrine synthesis of cortisol by the corneal epithelium contributes to immunoprotection. Primary cultures of human corneal epithelial cells, fibroblasts and allogeneic macrophages (M1) have been shown to be capable of generating cortisol (fibroblasts more so than epithelial cells) [727].
3.3.2 Glucocorticoid effects on the lacrimal gland
The action of GC on ocular surface and adnexal tissues is concentration dependent. In experimental conditions low amounts of GC are essential for the maintenance of rat lacrimal gland acinar epithelial cells in vitro [364], while physiological levels of GCs increase acinar cell synthesis of proteins such as the C3 component of prostatic binding protein, a potent immunosuppressive agent, and cystatin-related protein [381,647] as well as enhancing the androgen-induced production of secretory component [364]. Higher concentrations of GC promote C-reactive protein and C3 component of prostatic binding protein synthesis [381,647], but have no effect on basal secretory component output, and suppress the androgen related secretory component response [364,378]. The mechanism of action of GC on the lacrimal gland may depend on the presence of specific receptors since a glucocorticoid receptor mRNA was identified in human lacrimal tissue [733].
3.3.3 Glucocorticoids and sexual dimorphism
Since inflammation is a core component of a variety of sexually dimorphic diseases, such as autoimmunity, it was postulated that GC exert their anti-inflammatory effects in a sexually dimorphic manner and this may be the underlying mechanism by which men and women respond differently to inflammation [734]. The mechanisms through which GC exert their actions in a sexually dimorphic manner are complex. GC and estrogen receptors can interact and modulate downstream signaling, for example estrogens antagonize GC induction of the glucocorticoid-induced leucine zipper (GILZ) gene, an important mediator of GC anti-inflammatory actions. Hence, estrogen antagonism of GC receptor signaling could be responsible for the less efficient response of females to corticosteroid therapy [735]. A further possibility is a differential expression of sex-specific coactivators and transcription factors. For example females and males express different coregulatory molecules: prohibitin 2 and mediator complex subunit 12 are more expressed in females, while TATA box–binding protein is more expressed in males [734–736].
Notably, it appears that GC exert their anti-inflammatory effects more potently in males than in females, suggesting that synthetic GC might not be the most efficacious treatment paradigm for chronic inflammatory diseases in women. Thus, more personalized treatment strategies taking into account sexual dimorphism may need to be considered.
3.4 Regulation of the ocular surface and adnexa by hormones from the hypothalamic-pituitary axis
The hypothalamic-pituitary axis is the master regulator of the endocrine system. The hypothalamus processes signals from the central nervous and peripheral endocrine systems, and translates these inputs into signals to the anterior and posterior pituitary (hypophysis) gland. The pituitary then releases hormones that influence multiple tissues, and most endocrine organs, in the body and the hormones that mediate these regulatory functions include prolactin-releasing factor, thyrotropin-releasing hormone, corticotropin-releasing hormone, growth hormone-releasing hormone, gonadotropin-releasing hormone, thyroid-stimulating hormone, growth hormone, adrenocorticotropic hormone, follicle-stimulating hormone, and luteinizing hormone (Fig. 4).
Fig. 4 Hormones from the hypothalamic-pituitary axis. Abbreviations: PRF – prolactin-releasing factor; TRH – thyrotropin-releasing hormone; CRH – corticotropin-releasing hormone; GHRH – growth hormone-releasing hormone; GnRH – gonadotropin-releasing hormone; TSH - thyroid-stimulating hormone; GH – growth hormone; ACTH - adrenocorticotropic hormone; FSH - follicle-stimulating hormone; LH - luteinizing hormone.
As concerns the eye, hypophyseal hormones exert a significant influence on the ocular surface and adnexa. They are best known to modulate the growth, differentiation and function of the lacrimal gland [360,616,737–740], and to play a direct or indirect (e.g. regulation of sex steroid levels) role in promoting the sexual dimorphism of lacrimal tissue [91,616]. The actions of the hypothalamic-pituitary axis in general, and specific pituitary hormones in particular, on ocular surface and adnexal tissues in health and disease are described in this section. The effects of growth hormone are described in Section 3.5.
3.4.1 Impact of interrupting the hypothalamic-pituitary axis
Most information concerning the impact of the hypothalamic-pituitary axis on the ocular surface and adnexa relates primarily to the lacrimal gland and tear film. These data were obtained in animal studies following hypophysectomy, selective anterior pituitary ablation, or interruption of the hypothalamic-pituitary connection, as well as in dwarf mice with deficient pituitary function. These conditions cause a functional castration and reportedly erase sex-associated differences in the lacrimal gland and induce cytoplasmic vacuolar metamorphosis, nuclear pyknosis, acinar epithelial cell contraction, glandular atrophy, enhanced sulphate uptake, reduced tissue protein, total RNA and mRNA, decreased fluid and protein secretion and a reduced tear volume [12,13,87,91,100,204,360,378,417,616,741–745]. The decline in lacrimal gland weight in hypophysectomized animals (e.g. male rats) seems to be the consequence of an overall reduction in body weight, because the LGW/BW ratios in these animals are typically unchanged [204]. Further, whereas acinar epithelial cells may contract following anterior pituitary disruption, the density of acinar complexes may increase, but changes can be species specific [204]. The extent of lacrimal gland alterations following interruption of the hypothalamic-pituitary axis is often significantly greater in males than in females [91,743]. In addition, the influence of pituitary dysfunction on lacrimal tissue appears to be attributed to a loss of anterior, but not posterior, lobe hormones [408,410,411,616]. It is likely that the ability of pituitary extracts to increase LGW in guinea pigs [746] is due to hormones from the anterior pituitary. Such extracts also promote the proliferation of human meibomian gland epithelial cells [747]. Indeed, investigators reported that treatment of patients with an anterior pituitary extract attenuated their DED [410,411]. For comparison, the administration of posterior pituitary hormones to rabbits had no impact on lacrimal gland secretion [408].
Of particular interest, many but not all [745] androgen effects on the lacrimal gland are critically dependent upon an intact hypothalamic-pituitary axis. For example, acute or chronic testosterone administration has no consistent influence on the tear volume or protein concentration in orchiectomized animals that had undergone anterior pituitary ablation, hypophysectomy or pituitary transplant to the kidney capsule [12,204,412]. Similarly, androgens do not affect the weight, morphology, LGW/BW ratio or fluid secretion of lacrimal glands in pituitary-deficient animals [12,204,412,417,616], and their control of the lacrimal secretory immune system in vivo is almost completely inhibited by prior anterior pituitary ablation or hypophysectomy [529]. The mechanism(s) by which pituitary removal interferes with androgen action on the lacrimal gland may be complex [366,378]. Some of the underlying factors may be the significant decrease in the androgen receptor protein expression in acinar epithelial cell nuclei [97], as well as the significant reduction in androgen-induced transcriptional and post-transcriptional events in lacrimal tissue, following hypophysectomy [91].
However, if the pituitary is transplanted to the kidney capsule, androgen therapy increases the acinar complex area in lacrimal glands of hypophysectomized rats and delays the loss of LGW [204]. Further, treatment of hypophysectomized rats with testosterone and insulin significantly increases the LGW and the LGW/BW ratio [204]. The mechanism(s) underlying these androgen actions remain to be determined.
The impairment of lacrimal gland responses to androgens in pituitary-deficient animals does not represent a generalized lack of tissue responsiveness throughout the body. Testosterone administration to rats without anterior pituitaries induced a 20-fold increase in both the seminal vesicle weight (SVW) and SVW/BW ratio [204]. Thus, the influence of interrupting the hypothalamic-pituitary axis on androgen target organs appears to be site-specific.
3.4.2 Effects of hypothalamic-pituitary hormones
Anterior pituitary hormones, either directly or indirectly (e.g. through control of steroid, thyroxine and insulin), modulate the growth, differentiation and secretion of the lacrimal gland [360,410,411,514,616,737–740,742,748–750], as well as meibomian gland function [751]. The effects of growth hormone are described in Section 3.5.
3.4.2.1 Prolactin
Investigators have reported that prolactin increases the acinar cell diameter, nuclear size, and lacrimal gland weight of male and female dwarf mice [616], promotes the Na+,K+-ATPase and cholinergic receptor activities in lacrimal tissues of hypophysectomized female rats [360], and decreases carbachol-induced secretion by acinar epithelial cells [749,752,753]. In addition, prolactin has been proposed to play a minor role in the sexual dimorphism of murine lacrimal glands [748]. However, prolactin has also been demonstrated to exert no effect on the morphology (i.e. in hyperprolactinemic male mice) [748] and weight (i.e. in hypophysectomized male rats) [204] of lacrimal tissue, the synthesis and secretion of proteins by the lacrimal gland [366,752], and the volume of tears [204]. Also, exposure to increased prolactin levels, induced by metoclopramide treatment, leads to a structural disorganization of the mouse lacrimal gland [754].
The origin of lacrimal gland prolactin is not solely the pituitary. Prolactin and its receptor are transcribed and translated in lacrimal gland acinar epithelial cells [750,752,753,755–761]. Prolactin is also secreted by the lacrimal gland into tears [757,762]. It is unclear, though, what factors may modulate the synthesis and secretion of intra-lacrimal prolactin. Treatment with prolactin, prolactin antagonists (i.e. bromocriptine), or estrogen has no effect on lacrimal gland prolactin levels [763], and the administration of cholinergic agonists (i.e. pilocarpine) does not alter the concentration of prolactin secreted by lacrimal tissue [762]. It is possible that lacrimal prolactin could be regulated by androgens, given that the synthesis of this hormone and its receptors are regulated by androgens in other sites [764–767].
In summary, the species-independent role of prolactin in lacrimal tissue and tear film dynamics is unknown. Researchers have found a strong negative correlation between serum prolactin levels and tear function [768]. Considering this hormone's pro-inflammatory actions [766,769] and its proposed role in the pathogenesis of Sjögren syndrome [770,771], it is quite possible that the lacrimal synthesis of prolactin may act to promote autoimmune disease in the lacrimal gland. Conversely, testosterone's ability to down-regulate the prolactin receptor gene in the lacrimal gland may be one mechanism by which androgens suppress inflammation in this tissue in Sjögren syndrome [351].
With regard to the meibomian gland, investigators have found that women, but not men, with seborrheic MGD have significantly elevated serum levels of prolactin [772]. The reason for this linkage is unknown. Prolactin fragments, in turn, have been reported to inhibit corneal angiogenesis [773].
3.4.2.2 α-melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH)
The melanocortins α-MSH and ACTH are involved in the control of constitutive proteins by the lacrimal gland. These hormones have receptors on acinar epithelial cells of the rat lacrimal gland [738,740,774–777] and may stimulate cAMP production and protein release [737,738,740,778,779]. However, α-MSH and ACTH do not influence the output of all (e.g. regulated) lacrimal gland proteins [366].
When combined with androgens, α-MSH may increase lacrimal gland weight in orchiectomized rats [417]. Androgen treatment also up-regulates the lacrimal gland gene expression for the melanocortin 3 receptor [351], which may enhance the α-MSH- and ACTH-induced protein secretion [738,740]. In contrast, α-MSH has no effect on the relative (i.e. LGW/BW ratio) or absolute LGW [204,417], interactions between lacrimal acinar epithelial cells and lymphocytes [780], or the IgA content [741] or volume [204] of tears. Further, ACTH has no effect on acinar cell or nuclear diameters in lacrimal glands of pituitary-deficient mice [616].
Investigators have found that topical α-MSH application promoted the volume and stability of tears, improved corneal integrity and suppressed ocular surface inflammation in rats with scopolamine-induced DED. These α-MSH effects could be prevented by pharmacologically blocking either the PKA-CREB or MEK-Erk pathways [781].
Of interest are three additional studies concerning ACTH. First, ACTH may be synthesized by, or accumulate within, myoepithelial cells in the lacrimal gland [755]. Second, circulating ACTH levels have been positively correlated with central corneal thickness [782]. And third, an ACTH insensitivity syndrome (e.g. Allgrove) has been described, which is characterized by adrenal insufficiency, glucocorticoid deficiency and alacrima [783–789]. The reason for the ACTH-cornea association may relate to this peptide hormone's ability to influence corneal regeneration [790] and the mitotic index of corneal epithelium [791]. However, the consequence of lacrimal ACTH synthesis, and the specific cause of the decreased tear output in Allgrove syndrome, remains to be determined.
3.4.2.3 Thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and vasopressin
TSH, FSH and LH all increase the weight and differentiation of lacrimal glands in male, but not female, pituitary dwarf mice [616]. The reason for these sex-related differences in hormone action has yet to be clarified. Also to be determined is whether these pituitary hormone effects are direct (e.g. through lacrimal gland receptors), and/or are mediated through the regulation of other hormones (e.g. sex steroids, thyroxine). This question is very relevant for patients with hypothalamic hypogonadism, which was found associated with obstructive MGD in a 35-year-old male patient [792]. This condition is characterized by an impaired pituitary secretion of FSH and LH, leading to hypogonadism and a lack of sex steroid production. The authors of that case report proposed that this lid condition was due to androgen deficiency [792].
TSH receptors have also been identified in the human lacrimal gland [793]. These receptors are thought to be the target for autoantibodies in thyroid-associated ophthalmopathy, and, possibly through aberrant signal transduction, contribute to the reduced aqueous tear secretion and ocular surface damage in this disorder [793]. TSH levels in the serum of women, but not men, are also increased in seborrheic MGD [772]. The basis for this correlation is not known.
Vasopressin may be synthesized or accumulated by lacrimal gland epithelial cells [794], but the local effect of this hormone is unknown. Similarly, the potential effects of other hypothalamic and pituitary hormones on the ocular surface and adnexa have yet to be identified.
3.5 Growth hormone, insulin-like growth factor 1 and insulin regulation of the ocular surface and adnexa
Growth hormone (GH), insulin-like growth factor (IGF-1), as well as insulin are anabolic promoters responsible for mitosis, tissue growth, differentiation and repair. These actions are crucial for the health and functions of lacrimal gland, meibomian gland and ocular surface tissues as detailed below. GH, also called somatotropin, is an anterior pituitary hormone with actions mediated by effects of IGF-1 (also called somatomedin) via endocrine, paracrine and/or autocrine actions. GH, IGF-1 and insulin are involved in metabolism of glucose, amino acids, DNA, lipids and proteins [795–797]. These events are regulated by their respective cell surface receptors, which activate signaling molecules in the cytoplasm, eventually leading to powerful regulation of gene expression that favors cell survival and proliferation [798].
3.5.1 GH, IGF-1 and insulin mechanisms of action, interrelationships, and influence on the ocular surface and adnexa
GH binds to pre-dimerized GH receptors located on cell plasma membranes, leading to activation of intracellular Janus-kinase 2 (JAK2) [799], a kinase that phosphorylates itself as well as the GH receptor [800]. JAK2 further activates Signal Transducers and Activators of Transcription (STATs), which, upon tyrosyl phosphorylation, dimerize and enter the nucleus to regulate the transcription of GH-specific genes including IGF-1 [801]. GH induces cells to secrete IGF-1, which amplifies and complements GH effects, extending its actions, potentially in all cell types. IGF-1 has also paracrine secretion. It has structural and functional similarity with IGF-2 which, however, is not regulated by GH [802]. It is believed that IGF-2 is primarily a growth factor of the fetus whereas IGF-1 acts in postnatal tissues. IGF-2 in relation to corneal wound healing is briefly discussed in Section 3.5.4. IGF-1 signaling is initiated with binding to membrane bound IGF-1R, which phosphorylates and activates insulin receptor substrate (IRS)-1, leading to the cascade of phosphoinositide 3-kinase (PI3K)/Akt pathway activation [803,804], that is an important regulator of cell cycle progression and cell survival.
Insulin exerts its action via binding to insulin receptor (IR), leading to activation of IRS/PI3K/Akt and MAPK pathways [805]. IGF-1 and insulin have molecular similarity and are able to cross-activate each other's receptors, which are also structurally similar, as well as a hybrid IGF-1R/IR [796,806]. However, the cross affinity for the receptor is 100–1000 times lower than that of the specific molecule, depending on cell type [807–809]. In spite of cross-reactivity, IGF-1 and insulin exhibit different actions. Overall, IGF-1 is more efficient in inducing DNA synthesis and mitosis and insulin is more efficient in promoting metabolic events, potentially due to effects on different target cells. GH, IGF-1 and insulin receptors and their respective signaling pathways have been found in lacrimal gland and ocular surface tissues [364,810–815], and there is evidence of influences on tissue development, and wound healing responses.
3.5.1.1 Cornea
There is evidence that central corneal thickness (CCT) is decreased in GH deficient children and adults [782,816–818], and that GH treatment for one year increases CCT in GH deficient children [819]. However, others found no significant difference in CCT but increased corneal resistance factor (CRF) and corneal hysteresis (CH) [820]. CCT is also reported to be increased in acromegalic patients [816,821], but others observed no difference in CCT or corneal biomechanical parameters in acromegalic patients compared to age and sex matched controls [822]. High IGF-1 correlates with increased central corneal thickness in polycystic ovarian syndrome patients [823].
Insulin is present in the human tear film and insulin and IGF-1 receptors are found on the human ocular surface [811]. On the other hand, increased IGF-binding protein 3 is found in human tears, which may attenuate IGF-1R signaling in the diabetic cornea [824]. This may contribute to epithelial compromise and the pathogenesis of ocular surface complications reported in diabetes [824]. Insulin has also been found to promote corneal wound healing and may be of therapeutic benefit in delayed corneal wound closure associated with diabetes (see Section 3.5.4).
3.5.1.2 Meibomian gland
The GH/IGF-1 axis may positively regulate meibomian gland growth and function. For example, mouse meibomian gland size is positively correlated with GH and IGF-1 levels in a series of transgenic and knockout mouse lines that represent a spectrum of GH/IGF-1 excess, GH/IGF-1 deficiency and GH/IGF-1 absence [118]. Further, the meibomian gland shows normal morphology in GH/IGF-1 excess mice, but increased abnormalities including hyperkeratinized and thickened ducts, acini inserting into duct walls, and poorly differentiated acini in GH/IGF-1 deficient or absent mice [118]. At the cellular level, IGF-1 activates AKT but not ERK pathway, promotes cell proliferation and intracellular lipid accumulation in cultured human meibomian gland epithelial cells, whereas GH does not have a direct effect in vitro [751,825]. These data indicate that GH may exert an indirect effect on the meibomian gland via inducing IGF-1, however further research is needed.
Insulin, similar to IGF-1, activates AKT signaling via the IGF-1 receptor and promotes cell proliferation and lipid accumulation in cultured human meibomian gland epithelial cells [826]. Type II diabetes has been associated with MGD in some studies [116,827]. It is plausible that such an association may be mediated by toxicity of high glucose to meibomian gland epithelial cells, reducing IGF-1 receptor levels as well as other insulin and IGF-1 downstream signaling molecules [826].
3.5.1.3 Lacrimal gland
Insulin is itself secreted by the lacrimal gland acinar cells and it was demonstrated in rodents that this hormone is locally produced [828,829]. Insulin promotes tissue maintenance and supports constitutive secretion of elements present in the tears, as revealed in lacrimal gland culture studies [364,774,830]. Increasing the concentration of insulin, transferrin and selenium in the culture media of rat lacrimal gland acinar cells, induces secretion of secretory component, a protein that binds and transports IgA [364]. It also increases the synergic secretagogue effect of DHT [364]. In streptozotocin induced animal models of diabetes mellitus (DM), the loss of insulin results in smaller lacrimal glands with altered morphology of the secretory vesicles and intracellular vesicle trafficking, decreased corneal innervation and tear volume, and reduced concentrations of peroxidase in tears and IgA in the lacrimal gland [13,831–834]. The absence of insulin also increases the expression of oxidative markers such as malondialdehyde, peroxidase, and pro-inflammatory cytokines [831,835,836].
3.5.2 GH, IGF-1 and insulin roles in sex-related differences
3.5.2.1 Systemic sex-related effects of GH, IGF-1 and insulin
GH is well known to have a different secretion profile in men and women, resulting in vast differences in liver gene expression patterns [837,838]. Sex steroids modulate GH directly and indirectly via IGF-1 [839–841]. Insulin resistance is observed in postmenopausal women receiving oral but not transdermal combined estrogen and progesterone replacement therapy [842]. A possible explanation would be the inverse relationship between insulin binding sites and the levels of estradiol and progesterone during the menstrual cycle of young women and also lower insulin binding levels comparing those phases to young men, suggesting that sex hormones influence the levels of insulin receptor in target tissues (e.g. monocytes) [843]. This is relevant because it may link higher levels of sex hormones to lower action of insulin on the ocular surface and lacrimal gland. This linkage may help explain the stimulus for DED symptoms during the peaks of sex hormones in the follicular and luteal phase of the menstrual cycle and in polycystic ovary syndrome [313,608,842,844,845]. Overall, sex hormones play a significant role in modulating the activities of GH, IGF-1 and insulin.
3.5.2.2 Sex-related effects of GH, IGF-1 and insulin on the ocular adnexa
3.5.2.2.1 Lacrimal gland
Neither sex nor the phase of the estrous cycle change insulin receptor levels or early signaling steps in the rat lacrimal gland [73]. The number of androgen receptor–containing cells in lacrimal gland of rats and BALB/c or C57BL/6 mice were higher in male compared to female [97]. Streptozotocin-induced DM did not reduce the number of androgen receptor-containing cells in the rat lacrimal gland [97].
Non-obese diabetic mice (NOD) are widely used in research related to type 1 DM and Sjögren syndrome [846]. This animal model spontaneously created after hyperglycemic sibling inbreeding for several generations, presents a sex biased distribution of manifestations. NOD females are about 80% more frequently affected by DM and sialoadenitis and males are more affected by dacryoadenitis [65,847]. These sex-related differences are attributed to sex hormones [524,848].
Since the early steps of insulin signaling are similar in male and female lacrimal gland the sex hormone-driven changes in insulin, GH or IGF-1 signaling/effects are possibly occurring in later steps or at transcriptional levels of the signaling networks as observed in the modulation of MAP kinase or STAT by sex hormones in cultured cells [849,850]. In support of this hypothesis, testosterone treatment of orchiectomized mice altered the expression of insulin receptor-related receptor and insulin-like growth factor binding protein 3 [351]. In meibomian gland immortalized cell cultures, exposure to DHT increased the expression of genes related to insulin signaling [560]. The differences in gene expression in both studies include genes related to development, growth, metabolism, transport and other common actions associated with insulin, IGF-1 and GH, confirming their interactions with sex hormones.
3.5.2.2.2 Meibomian gland
Wild type female mice have larger meibomian glands than males, but there is no difference in size between male and female mice that are GH deficient [118]. It is possible that the sexually dimorphic GH effect is responsible, however it is not clear how this interaction of sex and GH affects meibomian gland function or MGD. Both GH and IGF-1 receptor mRNAs are expressed in the mouse [547] and human meibomian gland [110,560], where IGF-1 directly stimulates human meibomian gland epithelial cell proliferation and lipid accumulation [751], and mice show positive correlation in meibomian gland size with GH/IGF-1 activities [118].
3.5.3 Clinical relevance of GH, IGF-1 and insulin in DED
Acromegaly is a disease with overexpression of IGF-1 and consequently also higher secretion of IGF-1 with changes in the body structure and systems. An evaluation of 59 patients with acromegaly compared to 62 age and sex-matched controls revealed a slightly lower tear film breakup time (9.1 ± 3.6 vs. 10.7 ± 2.9, p = 0.009), but no differences in Schirmer test or tear film osmolarity, and no clinical differences in DED exams, despite levels of GH and IGF-1 that were on average more than double [851]. Dwarfism, a clinical condition marked by short stature, that is sometimes secondary to GH deficiency, has not been reported to be associated with DED. Gigantism, which is caused by overproduction of GH, has also never been associated with clinical findings of DED.
Pituitary deficiency has been associated with Sjögren syndrome and its clinical manifestations, including DED [852]. The expression of insulin receptor was increased while IGF-1 receptor was decreased, in minor salivary gland (MSG) tissues of Sjögren syndrome patients compared to controls [853]. Interestingly, IGF-1 itself has been found to co-localize with lymphocyte infiltration in Sjögren syndrome salivary glands, suggesting that IGF-I may be a target of autoimmunity in Sjögren syndrome [854].
Several clinical conditions are associated with insulin resistance and DED or DE symptoms. Among them are polycystic ovary syndrome, pregnancy, anti-androgen therapy and complete androgen insensitivity syndrome [10,540,608,855–857]. Interestingly they also present with changed levels or impaired action of sex hormones, in a complex manner with no clear cut cause-effect relationship with insulin resistance [858,859]. Moreover, DED is the most common side effect of figitumumab, an IGF-1 receptor blocking anti-cancer drug, in healthy human subjects. [860] The development of DED following IGF-1 receptor blockade may be due to disruption of meibomian gland or lacrimal gland function, and/or corneal innervation.
DED is associated with aging, and aging is accompanied by reduced levels of sex hormones and increased insulin resistance [19,861]. Aging in insulin-resistant rats shows increased oxidative stress and impaired vesicular transport, reduced tear flow, and higher levels of pro-inflammatory cytokines and other markers of tissue degeneration in the lacrimal gland, but normal levels of insulin in tears [862–864]. On the other hand, caloric restriction, a strategy known to retard the progress of aging and degenerative diseases in animals, reduced Sjögren syndrome related phenotypes in animal models, via reduction of insulin secretion and action [865–868]. With regard to corneal innervation, IGF-1 treatment not only accelerates corneal nerve regeneration, but also relieves DED in rabbits post LASIK surgery [869].
3.5.3.1 Diabetes and DED
3.5.3.1.1 Experimental data
Type I diabetes is known to cause DED [870], which can be secondary to autoimmune destruction of the lacrimal gland due to antigen cross activity with the pancreas [871]. DED secondary to autoimmunity has a similar distribution between the sexes and it is controversial as to whether metabolic factors play a role in type 1 diabetes-induced DED. Several animal studies have shown that insulin can restore lacrimal gland morphology, arguing for a metabolic or hormonal role of lacrimal gland dysfunction in diabetes. In streptozotocin-induced diabetic rats, the lacrimal gland shows reduced number and enlarged size of secretory vesicles, and insulin treatment restores the density of these vesicles [833]. Further, in this rat model of diabetes, changes in morphology, increased numbers of lipofuscin-like inclusions, increased malonaldehyde and total peroxidase activity were observed in the lacrimal gland, and these abnormalities were removed by insulin treatment [836]. In addition, expression of advanced glycation end products and their receptor, which are related to hyperglycemia, are increased in lacrimal glands of streptozotocin-induced diabetic rats [835]. Therefore even in type I diabetes, lacrimal gland dysfunction has a significant hormonal and metabolic component, plus a possible cross-reactive autoimmune component.
In contrast, type II diabetes associated DE is most likely hormonal or metabolic in nature. This disease, initiated by defective insulin action and hyperglycemia, decreases the microvascular, neural and metabolic integrity of the ocular surface, lacrimal gland and meibomian glands. It is possible that a direct effect of insulin via the IGF-1 receptor, and adverse signaling events caused by high glucose levels, on meibomian gland epithelial cells [826], may be involved in the MGD caused by diabetes.
3.5.3.1.2 Clinical data
Clinical ocular surface manifestations of diabetes include lower corneal sensitivity, lower tear film break up time and Schirmer test, epithelial metaplasia, and changes in tear proteins, and are reported to worsen with longer duration of disease and poor glycemic control [872–875]. Tear film osmolarity is higher in DM, compared to other causes of DED, and that may relate to a higher blood osmolarity reflecting a poor glycemic control [876]. This last conclusion is supported by an observation in which individuals with higher blood osmolarity were more prone to DE symptoms [877].
3.5.4 GH, IGF-1 and IGF-2, and insulin on corneal wound healing and neurotrophic keratitis
GH is known to promote skin epithelial wound healing [878–882]. In fact, it is often used off-label to promote skin healing associated with burns and surgery [878]. These observations led investigators to hypothesize that GH may also play a role in corneal epithelial wound healing and/or corneal nerve regeneration [815,883]. GH has been shown to promote corneal epithelial cell migration independent of IGF-1 in vitro [815]. More studies need to be performed to confirm positive effect of GH on corneal wound healing and/or nerve regeneration in vivo. If true, this may be beneficial in the treatment of corneal epithelial defects and/or neurotrophic keratitis associated with severe DED.
IGF-1 has been studied more extensively in terms of corneal wound healing, and found to promote corneal wound healing at multiple levels. First, IGF-1 promotes the proliferation and migration of corneal epithelial cells and fibroblasts [884–888], differentiation of limbal stem cells [889,890], and the proliferation of corneal endothelial cells in vitro [891–893]. Second, IGF-1 accelerates corneal epithelial migration and wound healing in organ culture [894–896] and animal models [897–900]. Third, IGF-1 also preserves corneal nerves in diabetic animals [890] and animals undergoing LASIK [869]. Lastly, in some human studies, IGF-1 has been found to prevent superficial punctate keratopathy in diabetic patients post cataract surgery [901], and accelerate reepithelialization in patients with neurotrophic keratopathy [902–904]. A summary of the findings of the role of IGF-1 in corneal wound healing is provided in Table 8 [869,884–913]. Insulin has been found to duplicate IGF-1 in promoting corneal wound healing in cell culture, organ culture and diabetic animal models of corneal wound healing. These findings are summarized in Table 9 [911,914–930].
Systemic replacement and/or usage of topical insulin treatment can reverse the signs of DED and wound healing defects in animal models of DM [836,920,931,932]. Autologous serum, which also contains insulin and growth factors has been used to attenuate the signs and symptoms of severe DE and delayed wound healing not just in DM, but also in several conditions associated with DE [933–935]. (See also TFOS DEWS II Management and Therapy Report) [936]. Different formulas and delivery systems of insulin and IGF-1 eye drops have been developed for DE treatment and a novel delivery system has been proposed to improve stability and the target tissue concentration of insulin [937–939].
IGF-2 has been demonstrated to act via IGF-1R in human embryonic corneal endothelial cells and stimulate their proliferation [940]. IGF-2 is also present in bovine corneal stroma, and stimulates proliferation of cultured keratocytes [941]. Similar to IGF-1 and insulin, IGF-2 also activates AKT signaling and promotes cell proliferation in cultured human corneal epithelial cells [915]. IGF-2R may play an active role in corneal wound healing. For example, IGF2R protein expression is increased during corneal wound healing in mouse corneas and IGF2R regulates human corneal fibroblast to myofibroblast differentiation [942]. IGF-2 also showed elevated expression after corneal injury and may facilitate limbal stem cell differentiation in corneal wound repair in a mouse model of mechanical cornea injury [943].
3.6 Thyroid hormone regulation of the ocular surface and adnexa
The thyroid gland secretes two hormones, triodothyronine (T3) and thyroxine (T4). Ocular and adnexal tissues are targets of thyroid hormones (TH), which have anabolic effects and promote lacrimal gland and other exocrine gland activity [944–947]. Thyroid gland diseases or thyroid hormone (TH) imbalance have negative effects on the lacrimal gland, tear film and ocular surface [948,949]. The causes may be lower hormone inputs to the ocular and adnexa tissues, contiguous inflammatory disease, or higher exposure of the ocular surface due to eyelid wide opening [950–953]. Specifically, Graves' ophthalmopathy, in its early phase is 5-fold more frequently associated with DED than healthy controls. The clinical findings observed in this recent report were lower Schirmer test, lower TBUT with fluorescein, and lower corneal sensitivity, with or without exophthalmos [954].
3.6.1 Thyroid hormone influence and mechanism of action
TH promote lipid and protein synthesis, tissue growth and differentiation [946]. The lacrimal gland and ocular surface epithelia have nuclear receptors for thyroid hormones and therefore are target organs for those hormones. Decreases of T3 and T4 induce hypotrophy of the lacrimal gland, cornea epithelia metaplasia and reduced tear flow [399,944,955].
TH work primarily through nuclear receptors, which promote the expression of specific genes. The two isoforms of the thyroid hormone receptors, alpha and beta, are further divided in types 1 and 2. They are differently expressed in different tissues [956]. Thyroid hormone receptor beta-1 is expressed in rat lacrimal gland and hormone reduction for ten weeks induces its up-regulation in this tissue [944]. TH are well known to influence lipolysis and lipogenesis; their deficiency causes hypercholesterolemia and reduction of lipid secretion by sebaceous glands [946,957], suggesting that TH may also exert influence on the meibomian glands.
3.6.2 Thyroid hormone role in sex-related differences
The frequency of autoimmune thyroid diseases, Hashimoto's thyroiditis and Graves' disease are several times higher in females than males [958]. Moreover, Sjögren syndrome, a disease ten times more frequent in females is commonly associated with thyroid-associated diseases [948,951]. These events taken together indicate that female sex and/or female sex hormones predispose to inflammatory events in the thyroid gland and related target tissues. In addition, TH imbalance may contribute to the development of autoimmune diseases.
3.6.3 Clinical relevance of thyroid hormone action in DED
Several diseases related to the thyroid gland and its hormones may affect the ocular surface and induce DED. The three major mechanisms are mechanical (proptosis associated with Graves' disease), autoimmunity that extends to ocular and adnexal tissues; and TH loss in conditions related to iodine scarcity, thyroid radiotherapy, thyroid gland ablation and poor hormone replacement in different diseases.
Thyroid-associated ophthalmopathy features DED manifestations, including symptoms, low tear secretion, low TBUT, punctate keratitis, rose bengal staining, higher tear osmolarity and MGD [793,959]. A challenging aspect is that no single test is capable of distinguishing patients with DED due to Graves' ophthalmopathy in a population setting [876]. A recent study using proteomics to compare the profile of tears from patients with Thyroid-associated orbitopathy (TAO), DED patients without TAO and healthy controls revealed that a similar profile in TAO with or without DED, compared to DED and control groups [466]. Although the work did not clearly define the criteria to label DED individuals or the possible causes of DED in the non TAO group, it indicated a higher expression of pro-inflammatory proteins in both TAO groups, suggesting possible biomarkers and unique mechanisms of disease in DED caused by TAO [960].
4. Gender, health, and DED back to top
4.1 Sex and gender as interrelated distinct characteristics
Animal studies reveal sex-based differences that are rooted in biology and appear to affect risk for DED and could shed light on the mechanisms behind the disease and its presentation [89,98,263,265,527,671]. For example, Zylberberg et al. [671] found increased levels of the pro-MMPs-2 and -9 in cell cultures obtained from female rabbits' lacrimal glands that were exposed to estradiol but not to DHT. Both of these MMPs are found in increased concentrations in tear fluids from patients with DED, suggesting that these MMPs may be implicated in the pathogenesis of this disease. In addition, Seamon et al. [98] demonstrated reduced levels of tear lipocalin in ovariectomized rabbits, adding to the evidence linking reduced levels of sex steroids with DED—a finding that might help explain why postmenopausal women are at increased risk of DED (see Section 2.2.3).
When considering health and disease and the provision of health care, gender—the socially constructed differences between men and women that give rise to conceptions of masculinity and femininity—must also be taken into account [961]. Various factors related to sex and gender place women at heightened risk for DED and make them vulnerable to disparities in care and outcomes. The literature abounds with examples of gender-based health disparities in access to care, care-seeking behavior (particularly in women in developed countries), communication with health care providers, service utilization, and health outcomes around the world, and these examples may likewise apply in the case of DED. Studies, for example, have documented disparities in colorectal cancer screening [962,963], access to screening and treatment for HIV infection [964,965], quality of life for epilepsy patients [966,967], referrals for cardiac rehabilitation [968], access to and care-seeking behavior for mental health services [969,970], quality of life and long-term outcomes of stroke survivors [971], and care provided for type 2 diabetes and in lower-extremity amputations among patients with diabetes [972,973].
According to Schiebinger and Stefanick [974] gender can be broken down into “gender identity” (how individuals and groups perceive and present themselves), “gender norms” (unspoken rules in the family, workplace, institutional, or global culture that influence individual attitudes and behaviors), and “gender relations” (the power relations between individuals of different gender identities). The many known determinants of women's eye health disparities include uneven access to care and treatment due to socioeconomic factors; attitudes and behaviors about preventive care; gender-based differences in health-seeking behaviors; age [33]. That biological age is a key risk factor for eye and vision problems, including DED, contributes to the notion that women, who live longer than men on average, also suffer significant disability from chronic vision conditions that predominate in older people [33]. In addition, many autoimmune diseases are more prevalent in women than in men [33]. Certain of these diseases (e.g. systemic lupus erythematosus, rheumatoid arthritis) are associated with DED (see Section 2.2.3.).
4.2 Gendered behaviors can lead to gender differences in eye conditions
Trachoma and onchocerciasis, the first and second leading causes of infectious blindness in the world, are classic examples of gender-based health issues. In the developing world, where trachoma is common, women are three times more likely than men to be blinded by trachoma, due to the influence of gender-defined roles for women [975]. Women have more physical contact with people who are infected, putting them at greater risk of exposure. Women are also less likely to seek and receive treatment for trachoma [33]. Onchocerciasis in contrast, disproportionately affects men compared to women. This imbalance is due to the influence of gender roles as well, with men in endemic areas spending more time than women in aquatic environments, such as polluted rivers, where the parasitic disease spreads via an insect vector [33].
Gendered behaviors also affect risk for DED in a significant way, as women may experience heightened attention to their cosmetic appearance, including the wearing of spectacles. For example, in certain countries more women wear contact lenses than men, and contact lens wear is associated with a heightened risk for DED stemming from lens use [976]. Nichols and Sinnott found that women who wore contact lenses were more likely to have DED than were men, with 40% of the men and 62% of the women in the study classified as having DED (P < 0.0001) [977]. Women are also more likely to undergo laser refractive surgery [978], which is associated with an elevated risk of developing DED [979–982]. In another study, the combination of oral contraceptive pill use and contact lens wear appeared to increase the severity of DED symptoms in young women [58,699,983].
4.3 Gender concordance between patient and care provider adds another dimension
A substantial body of literature addresses the influence of gender on the interactions between patients and their care providers. It has been postulated that the gender composition of the patient–clinician dyad could affect communication, shared decision-making, and other aspects of health care, but the answer is still not clear. For example, a patient-level meta-analysis by Wyatt et al. [984] across 7 clinical trials (775 clinical encounters) found no statistically significant interaction between clinician–patient gender mix and decisional conflict, satisfaction with the clinical encounter, or patient engagement. A borderline significant interaction was observed only for increased concordance between stated decision and action taken, for encounters involving clinicians who were women and patients who were men (p = 0.05). All other gender dyads showed decreased concordance (6% fewer concordant encounters for same-gender, 16% fewer concordant encounters for clinicians who were men/patients who were women) [984]. A systematic review of the literature undertaken by Deepmala et al. [985] found that 10 of 12 studies analyzed provided evidence that provider characteristics, including age, sex/gender, experience, and specialty, as well as the interplay between provider and patient characteristics are important variables in pain management with analgesics.
4.4 Sex and gender influence the experience and treatment of pain
Pain is a hallmark of DED. Surveys of epidemiologic and laboratory data as well as electronic medical records provide strong evidence for clinical and experimental sex and gender differences in pain [986,987]. Many studies show that men have higher pain thresholds than women. This relationship holds true even for children and adolescents [988,989]. Various explanations for this phenomenon have been given, ranging from experiential and sociocultural gender differences in pain experience between men and women to hormonally and genetically driven sex differences in brain neurochemistry [986]. Having lower pain thresholds and tolerances (i.e. greater sensitivity to pain) leaves women at particularly high risk of being undertreated for pain [990]. Health care providers should take into account the role of gender when assessing their patients' complaints of pain because men and women differ in their perception of pain, their tendency to complain of pain, and their methods for coping with pain [991,992].
Animal studies can help distinguish the relative contributions of biological sex and gender to pain and pain attenuation. Many studies in animal models have demonstrated sex-specific differences in responses to pain and pain attenuation. In a study of rats, Liu et al. [993] found a striking interdependence of sex and pain type that determines the manifestation of antinociception (reduction of sensitivity to painful stimulus) mediated by Dyn protein and its associated kappa opioid receptor (KOR). Both sex and pain type are important determinants of Dyn/KOR antinociception; neither variable acts independently of the other. Another group reported sex-specific differences in pain response by dopamine in the bed nucleus of the stria terminalis in rats [994,995]. Yet another study revealed increased heat sensitivity and decreased cold sensitivity for female rats, but not males that underwent injections of quisqualic acid into the thoracic gray matter or sham operations. This selective effect is indicative of altered sympathetic activation by the thoracic injections. The effect of sham surgery suggests that female rats are vulnerable to ischemic injury during exposure and manipulation of the spinal cord [996]. One study examining chronic pain among the elderly found that women tended to adopt more psychologic approaches, such as acceptance and ignoring to relieve pain, than men [997]. In a study of nearly 800 men and women with fibromyalgia syndrome, Racine et al. [991] found that men were more likely to view pain as evidence of harm, and they were also more likely than women to avoid activity as a way to cope. Curtailing activity as a pain-coping strategy appears to be detrimental to men's quality of life [998].
Moreover, the role played by care providers' gender in pain management cannot be ignored. A study involving 310 general practitioners revealed that evidence of pathology had a larger effect on referrals of low-back pain patients to psychology/psychiatry by physicians who were men than those who were women. Also, the gender of the clinician moderated the pain judgments that accounted for the effect of pathology findings and pain behaviors on prescribing patterns [999]. For example, physicians' gender had a significant impact on pain management decisions in patients with low back pain, according to a review of 186 medical records [1000].
Care providers may view subjective complaints differently than objective tests. In cases of DED, this is problematic because symptom complaints do not correlate well with ocular findings. Management of DED symptoms is complex, and health care providers need to consider a patient's holistic picture, rather than simply treating ocular signs. For example, Vehof et al. [293] conducted a cross-sectional study of 1622 twin volunteers, all of whom were women, ranging in age from 20 to 83. A total of 438 (27.0%) were categorized as having DED. Women with the disease had significantly greater pain sensitivity, lower pain tolerance, and more pain symptoms than those without DED, strengthening the evidence of associations between the severity of tear insufficiency, cell damage, and psychological factors.
5. Recommendations for future research related to sex, gender, hormones and DED back to top
DED can cause significant pain, but little is known about factors contributing to symptoms of DED, given the poor correlation between these symptoms and objective signs at the ocular surface [274,293]. The hope is that we can identify better ways to predict risk for DED and develop novel therapies to alleviate this condition with more targeted, mechanistic approaches instead of relying on nonspecific symptom relief. Sex, gender and hormones exert a significant influence on the ocular surface and adnexa, and play a significant role in the pathogenesis of aqueous-deficient and evaporative DED. However, further studies are required to clarify the precise nature, extent, and mechanisms of these sex, endocrine and gender effects on the eye in health and disease. Such studies need to:
• Use the terms sex and gender consistently and correctly across scientific disciplines, in order to promote the accurate assessment, measurement, and reporting of differences between men and women;
• Conduct more epidemiological studies on the prevalence of DED by using both sign and symptom data;
• Use the term sex in most studies of nonhuman animals;
• Include sex as a variable in basic and clinical research, and take donor sex into account in experiments with cultured cells;
• Select animal models for research that mirror human sex differences and are relevant for DED;
• Evaluate natural genetic variability, disorders of sex differentiation, reproductive status and environmental influences to gain a better understanding of human DED;
• Elucidate the roles of the sex chromosome complement (e.g. parent-of-origin effects, X-inactivation, and genes in the non-recombining region of the Y chromosome), sex-specific autosomal factors and epigenetics (e.g. miRNAs, DNA methylation and acetylation, and histone modifications), as well as the microbiome, in mediating sex-related differences;
• Develop systems that identify and differentiate the effects of genes from those of hormones;
• Determine the processes involved in the sex steroid, hypothalamic-pituitary hormone, glucocorticoid, insulin, IGF-1 and thyroid hormone regulation of ocular surface tissues, and how they contribute to the sex-related differences in DED;
• Perform clinical studies to determine whether a sexual dimorphism exists in the response to topical GCs for the treatment of inflammatory ocular surface disorders;
• Use functional neuroimaging (e.g. positron emission tomography, fMRI) to evaluate sex-related differences in the pain of DED, as well as in experimentally-induced pain stimuli to the ocular surfaces of healthy subjects;
• Develop innovative human experimental models that better mimic clinical pain in DED;
• Determine whether sex differences exist in the pattern of innervation, the capacity to release neurotransmitters, and the sensitivity to neural stimulation, in ocular surface and adnexal tissues;
• Determine whether sex differences are present in the levels of tear film biomarkers in health and disease, and whether these measures could be used diagnostically;
• Assess whether diagnostic tests and management of DED should be different in men and women;
• Examine the utility of local (i.e. intracrine) hormone measurements for the diagnosis of DED;
• Conduct clinical studies to determine the extent to which MGD, as defined by using meibomian gland diagnostic tests, shows sex-related differences;
• Determine whether the sex difference in DED lessens with more advanced age, becoming more similar among women and men;
• Communicate clearly about the role of sex and gender influences in the arenas of DED research, patient care, and health and science policy;
• Determine whether the use of cosmetics contributes to the gender-related differences in the prevalence of dry eye disease;
• Ensure adequate participation of women in clinical trials, and analyze data by sex/gender to determine differences in response to treatment.
6. Financial disclosures back to top
David Sullivan: Allergan (F), Novagali/Santen (F), Cempra (F), TearLab (F), GlaxoSmithKline (F), Singularis (I), M.G. Therapeutics (C), Dompé (R), Sanofi Fovea (R), Novartis (R), Laboratoire Théa (R), Lμbris (R), Eduardo Rocha: Johnson and Johnson Brazil (C), Pasquale Aragona: None, Janine Clayton: None, Juan Ding: None, Blanka Golebiowski: Tearlab (F), Minifab (F), Lawley Pharmaceuticals (F), Ulrike Hampel: Bausch and Lomb GmbH (F), Optima Phamazeutische GmbH (F), Alison McDermott: CooperVision (F), Debra A. Schaumberg: Mimetogen (I), Shire (SARcode) (C), Eleven Bio (C), Nicox (C), Santen (R), Sruthi Srinivasan: Advanced Vision Research (F), Alcon (F; C; R), AlgiPharma (F), Allergan (F), CooperVision (F), Essilor (F), Johnson and Johnson Vision Care (F; R), Ocular Dynamics (F), Oculus (F), Ocusense (F), TearScience (F), Visioneering Technologies (F), Shire (C), Piera Versura: None, Mark Willcox: Alcon Laboratories (F), Allergan (F), CooperVision (F), Johnson & Johnson Vision Care (F), Ophtecs (F); Allergan (C), Minomic International Pty. (C), Ophtecs (C), Warm Contacts (C); Allergan (R), Ophtecs (R); Minomic International Pty. (S), Ophtecs (S).
F (Financial Support), I (Personal Financial Interest), E (Employment), C (Consultant), P (Patent), R (Recipient), N (No Commercial Relationship), S (non-remunerative).
The authors would like to thank the following individuals: Florence Haseltine, for her insight into the importance of considering sex and gender as basic human variables; Wendy Kam, for her EndNote expertise; Yang Liu, for her extensive literature searches and summaries; and Amy Gallant Sullivan for her excellent translations of the entire 1905 Berger E & Loewy R. book (“Ocular troubles originating from the female genital tract”) [4] from French to English, and the Lagresa et al. [461] publication from Spanish to English. Thanks to Nino Longo (Catania, Italy) and Sabrina Zappia (Rome, Italy) for help with graphics and illustrations.
[1] The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop. Ocul Surf 2007;2007(5):93–107.
[2] Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005.
[3] Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences: Exploring the Biological Contributions to Human Health: Does Sex Matter?. Washington, DC: The National Academies Press; 2001.
[4] Berger E, Loewy R. In: Alcan F, editor. Ocular troubles originating from the female genital tract. Ancienne Libraire Germer Balliere et Cie, Paris. 1905.
[5] Kollock CW. Diseases and functional disorders of the eye, produced by normal and abnormal conditions of the sexual organs. Trans S C Med Assoc 1888:97–102.
[6] Suzuki T, Sullivan BD, Liu M, Schirra F, Richards SM, Yamagami H, et al. Estrogen and progesterone effects on the morphology of the mouse meibomian gland. Adv Exp Med Biol 2002;506:483–488.
[7] Chew CK, Hykin PG, Jansweijer C, Dikstein S, Tiffany JM, Bron AJ. The casual level of meibomian lipids in humans. Curr Eye Res 1993;12:255–259.
[8] Richards SM, Yamagami H, Schirra F, Suzuki T, Jensen RV, Sullivan DA. Sex-related effect on gene expression in the mouse meibomian gland. Curr Eye Res 2006;31:119–128.
[9] Suzuki T, Richards SM, Liu S, Jensen RV, Sullivan DA. Influence of sex on gene expression in human corneal epithelial cells. Mol Vis 2009;15:2554–2569.
[10] Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol 2002;120:1689–1699.
[11] Ranganathan V, De PK. Androgens and estrogens markedly inhibit expression of a 20-kDa major protein in hamster exorbital lacrimal gland. Biochem Biophys Res Commun 1995;208:412–417.
[12] Sullivan DA, Allansmith MR. Hormonal modulation of tear volume in the rat. Exp Eye Res 1986;42:131–139.
[13] Sullivan DA, Hann LE. Hormonal influence on the secretory immune system of the eye: endocrine impact on the lacrimal gland accumulation and secretion of IgA and IgG. J Steroid Biochem 1989;34:253–262.
[14] Cornell-Bell AH, Sullivan DA, Allansmith MR. Gender-related differences in the morphology of the lacrimal gland. Invest Ophthalmol Vis Sci 1985;26:1170–1175.
[15] Goto T, Klyce SD, Zheng X, Maeda N, Kuroda T, Ide C. Gender- and age-related differences in corneal topography. Cornea 2001;20:270–276.
[16] Imbert Y, Foulks GN, Brennan MD, Jumblatt MM, John G, Shah HA, et al. MUC1 and estrogen receptor alpha gene polymorphisms in dry eye patients. Exp Eye Res 2009;88:334–338.
[17] Yolton DP, Yolton RL, Lopez R, Bogner B, Stevens R, Rao D. The effects of gender and birth control pill use on spontaneous blink rates. J Am Optom Assoc 1994;65:763–770.
[18] Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003;136:318–326.
[19] Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol 2003;31:229–232.
[20] Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 2011;52:1938–1978.
[21] Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology 2000;107:1157–1163.
[22] Crabb CV. Endocrine influences on ulceration and regeneration in the alkali-burned cornea. Arch Ophthalmol 1977;95:1866–1870.
[23] Waltman SR, Burde RM, Berrios J. Prevention of corneal homograft rejection by estrogens. Transplantation 1971;11:194–196.
[24] Chiaroni-Clarke RC, Munro JE, Ellis JA. Sex bias in paediatric autoimmune disease - Not just about sex hormones? J Autoimmun 2016;69:12–23.
[25] Ostrer H. Invited review: sex-based differences in gene expression. J Appl Physiol 1985;2001(91):2384–2388.
[26] Disteche CM, Filippova GN, Tsuchiya KD. Escape from X inactivation. Cytogenet Genome Res 2002;99:36–43.
[27] Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain Res 2006;1126:50–55.
[28] Migeon BR. The role of X inactivation and cellular mosaicism in women's health and sex-specific diseases. JAMA 2006;295:1428–1433.
[29] Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet 2005;21:298–305.
[30] Isensee J, Ruiz Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med 2007;4(Suppl B):S75–S95.
[31] Khan D, Dai R, Ansar Ahmed S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell Immunol 2015;294:70–79.
[32] Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, et al. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47,XXX in systemic lupus erythematosus and Sjögren's syndrome. Arthritis Rheumatol 2016;68:1290–1300.
[33] Clayton JA, Davis AF. Sex/gender disparities and women's eye health. Curr Eye Res 2015;40:102–109.
[34] Brandt JE, Priori R, Valesini G, Fairweather D. Sex differences in Sjögren's syndrome: a comprehensive review of immune mechanisms. Biol Sex Differ 2015;6:19.
[35] Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol 2009;127:763–768.
[36] Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000;118:1264–1268.
[37] Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol 2014;157:799–806.
[38] Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol 1997;124:723–728.
[39] Malet F, Le Goff M, Colin J, Schweitzer C, Delyfer MN, Korobelnik JF, et al. Dry eye disease in French elderly subjects: the Alienor Study. Acta Ophthalmol 2014;92. e429–36.
[40] Viso E, Rodriguez-Ares MT, Gude F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study). Ophthalmic Epidemiol 2009;16:15–21.
[41] Guo B, Lu P, Chen X, Zhang W, Chen R. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthalmic Epidemiol 2010;17:234–241.
[42] Hua R, Yao K, Hu Y, Chen L. Discrepancy between subjectively reported symptoms and objectively measured clinical findings in dry eye: a population based analysis. BMJ Open 2014;4:e005296.
[43] Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23:688–693.
[44] Lu P, Chen X, Liu X, Yu L, Kang Y, Xie Q, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea 2008;27:545–551.
[45] Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol 2002;86:1347–1351.
[46] Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea 1999;18:408–411.
[47] Uchino M, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Takebayashi T, et al. Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol 2008;146:925–929. e2.
[48] Uchino M, Schaumberg DA, Dogru M, Uchino Y, Fukagawa K, Shimmura S, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008;115:1982–1988.
[49] Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011;118:2361–2367.
[50] Uchino M, Yokoi N, Uchino Y, Dogru M, Kawashima M, Komuro A, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol 2013;156:759–766.
[51] Tan LL, Morgan P, Cai ZQ, Straughan RA. Prevalence of and risk factors for symptomatic dry eye disease in Singapore. Clin Exp Optom 2015;98:45–53.
[52] Tong L, Saw SM, Lamoureux EL, Wang JJ, Rosman M, Tan DT, et al. A questionnaire-based assessment of symptoms associated with tear film dysfunction and lid margin disease in an Asian population. Ophthalmic Epidemiol 2009;16:31–37.
[53] Ahn JM, Lee SH, Rim TH, Park RJ, Yang HS, Kim TI, et al. Prevalence of and risk factors associated with dry eye: the Korea national health and nutrition examination survey 2010-2011. Am J Ophthalmol 2014;158:1205–1214. e7.
[54] Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH, Kim KW. Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol 2011;129:633–638.
[55] Um SB, Kim NH, Lee HK, Song JS, Kim HC. Spatial epidemiology of dry eye disease: findings from South Korea. Int J Health Geogr 2014;13:31.
[56] Lin PY, Cheng CY, Hsu WM, Tsai SY, Lin MW, Liu JH, et al. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2005;46:1593–1598.
[57] Schaumberg DA, Uchino M, Christen WG, Semba RD, Buring JE, Li JZ. Patient reported differences in dry eye disease between men and women: impact, management, and patient satisfaction. PLoS One 2013;8:e76121.
[58] Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology report. Ocul Surf 2017;15:334–365.
[59] Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol 2014;98:1712–1717.
[60] Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA 2001;286:2114–2119.
[61] Schaumberg DA, Li J. Gender differences in dry eye disease impact, management, patient satisfaction, and comorbid conditions (abstract). In: 6th International Conference on the Tear Film and Ocular Surface: Basic Science and Clinical Relevance. 2010. p. 106.
[62] Management and therapy of dry eye disease: report of the management and therapy subcommittee of the international dry eye workshop. Ocul Surf 2007;2007(5):163–178.
[63] Lienert JP, Tarko L, Uchino M, Christen WG, Schaumberg DA. Long-term natural history of dry eye disease from the patient's perspective. Ophthalmology 2016;123:425–433.
[64] Hunger RE, Carnaud C, Vogt I, Mueller C. Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice. J Clin Invest 1998;101:1300–1309.
[65] Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA. Impact of gender on exocrine gland inflammation in mouse models of Sjögren's syndrome. Exp Eye Res 1999;69:355–366.
[66] Toda I, Wickham LA, Sullivan DA. Gender and androgen treatment influence the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary tissues of MRL/lpr mice. Clin Immunol Immunopathol 1998;86:59–71.
[67] Bromberg BB, Welch MH, Beuerman RW, Chew SJ, Thompson HW, Ramage D, et al. Histochemical distribution of carbonic anhydrase in rat and rabbit lacrimal gland. Invest Ophthalmol Vis Sci 1993;34:339–348.
[68] Cripps M, Bromberg B, Welch M. Gender-dependent lacrimal protein secretion (abstract). Invest Ophthalmol Vis Sci 1986;27:25.
[69] Walker R. Age changes in the rat's exorbital lacrimal gland. Anat Rec 1958;132:49–69.
[70] El-Fadaly AB, El-Shaarawy EA, Rizk AA, Nasralla MM, Shuaib DM. Age-related alterations in the lacrimal gland of adult albino rat: a light and electron microscopic study. Ann Anat 2014;196:336–351.
[71] Gancharova OS, Manskikh VN. Age-related changes in the rat lacrimal gland: specific morphology and unknown nature. Ontogenez 2014;45:289–298.
[72] Sashima M, Hatakeyama S, Satoh M, Suzuki A. Harderianization is another sexual dimorphism of rat exorbital lacrimal gland. Acta Anat (Basel) 1989;135:303–306.
[73] Rocha EM, Hirata AE, Carneiro EM, Saad MJ, Velloso LA. Impact of gender on insulin signaling pathway in lacrimal and salivary glands of rats. Endocrine 2002;18:191–199.
[74] Huang Z, Lambert RW, Wickham LA, Sullivan DA. Analysis of cytomegalovirus infection and replication in acinar epithelial cells of the rat lacrimal gland. Invest Ophthalmol Vis Sci 1996;37:1174–1186.
[75] Sullivan DA, Colby EB, Hann LE, Allansmith MR, Wira CR. Production and utilization of a mouse monoclonal antibody to rat IgA: identification of gender-related differences in the secretory immune system. Immunol Invest 1986;15:311–325.
[76] Hann LE, Allansmith MR, Sullivan DA. Impact of aging and gender on the Ig-containing cell profile of the lacrimal gland. Acta Ophthalmol (Copenh) 1988;66:87–92.
[77] Gubits RM, Lynch KR, Kulkarni AB, Dolan KP, Gresik EW, Hollander P, et al. Differential regulation of alpha 2u globulin gene expression in liver, lachrymal gland, and salivary gland. J Biol Chem 1984;259:12803–12809.
[78] Haendler B, Toda I, Sullivan DA, Schleuning WD. Expression of transcripts for cysteine-rich secretory proteins (CRISPs) in the murine lacrimal gland. J Cell Physiol 1999;178:371–378.
[79] Huang Z, Gao J, Wickham L, Sullivan D. Influence of gender and androgen treatment on TGF-beta 1 mRNA levels in the rat lacrimal gland. Invest Ophthalmol Vis Sci 1995;1995:35. S991.
[80] Remington SG, Crow JM, Nelson JD. Secretoglobins: lacrimal gland-specific rabbit lipophilin mRNAs. Invest Ophthalmol Vis Sci 2008;49:2856–2862.
[81] Remington SG, Lima PH, Nelson JD. Pancreatic lipase-related protein 1 mRNA in female mouse lacrimal gland. Invest Ophthalmol Vis Sci 1999;40:1081–1090.
[82] Remington SG, Nelson JD. Secretoglobins: sexually dimorphic expression of androgen-binding protein mRNA in mouse lacrimal glands. Invest Ophthalmol Vis Sci 2005;46:31–38.
[83] Richards SM, Jensen RV, Liu M, Sullivan BD, Lombardi MJ, Rowley P, et al. Influence of sex on gene expression in the mouse lacrimal gland. Exp Eye Res 2006;82:13–23.
[84] Richards SM, Liu M, Sullivan BD, Sullivan DA. Gender-related differences in gene expression of the lacrimal gland. Adv Exp Med Biol 2002;506:121–127.
[85] Robinson CP, Cornelius J, Bounous DE, Yamamoto H, Humphreys-Beher MG, Peck AB. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity 1998;27:29–44.
[86] Sakulsak N, Wakayama T, Hipkaeo W, Iseki S. A novel mouse protein differentially regulated by androgens in the submandibular and lacrimal glands. Arch Oral Biol 2007;52:507–517.
[87] Shaw PH, Held WA, Hastie ND. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell 1983;32:755–761.
[88] Winderickx J, Hemschoote K, De Clercq N, Van Dijck P, Peeters B, Rombauts W, et al. Tissue-specific expression and androgen regulation of different genes encoding rat prostatic 22-kilodalton glycoproteins homologous to human and rat cystatin. Mol Endocrinol 1990;4:657–667.
[89] Rahimi Darabad R, Suzuki T, Richards SM, Jakobiec FA, Zakka FR, Barabino S, et al. Does estrogen deficiency cause lacrimal gland inflammation and aqueous-deficient dry eye in mice? Exp Eye Res 2014;127:153–160.
[90] Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis 2009;15:1553–1572.
[91] Gao J, Lambert RW, Wickham LA, Banting G, Sullivan DA. Androgen control of secretory component mRNA levels in the rat lacrimal gland. J Steroid Biochem Mol Biol 1995;52:239–249.
[92] Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol 2007;17:1879–1884.
[93] Lauria A, Porcelli F. Leucine aminopeptidase (LAP) activity and sexual dimorphism in rat exorbital lacrimal gland. Basic Appl Histochem 1979;23:171–177.
[94] Mhatre MC, van Jaarsveld AS, Reiter RJ. Melatonin in the lacrimal gland: first demonstration and experimental manipulation. Biochem Biophys Res Commun 1988;153:1186–1192.
[95] Paliwal A, De PK. Marked sexual dimorphism of lacrimal gland peroxidase in hamster: repression by androgens and estrogens. Biochem Biophys Res Commun 2006;341:1286–1293.
[96] Ranganathan V, Jana NR, De PK. Hormonal effects on hamster lacrimal gland female-specific major 20 kDa secretory protein and its immunological similarity with submandibular gland major male-specific proteins. J Steroid Biochem Mol Biol 1999;70:151–158.
[97] Rocha FJ, Wickham LA, Pena JD, Gao J, Ono M, Lambert RW, et al. Influence of gender and the endocrine environment on the distribution of androgen receptors in the lacrimal gland. J Steroid Biochem Mol Biol 1993;46:737–749.
[98] Seamon V, Vellala K, Zylberberg C, Ponamareva O, Azzarolo AM. Sex hormone regulation of tear lipocalin in the rabbit lacrimal gland. Exp Eye Res 2008;87:184–190.
[99] Srikantan S, De PK. Sex differences in expression and differential regulation by androgen and estrogen of two odorant-binding tear lipocalins in lacrimal glands of immature hamsters. Gen Comp Endocrinol 2008;158:268–276.
[100] Sullivan DA, Allansmith MR. Hormonal influence on the secretory immune system of the eye: androgen modulation of IgA levels in tears of rats. J Immunol 1985;134:2978–2982.
[101] Sullivan DA, Allansmith MR. The effect of aging on the secretory immune system of the eye. Immunology 1988;63:403–410.
[102] Sullivan DA, Bloch KJ, Allansmith MR. Hormonal influence on the secretory immune system of the eye: androgen control of secretory component production by the rat exorbital gland. Immunology 1984;52:239–246.
[103] Sullivan DA, Bloch KJ, Allansmith MR. Hormonal influence on the secretory immune system of the eye: androgen regulation of secretory component levels in rat tears. J Immunol 1984;132:1130–1135.
[104] Vercaeren I, Vanaken H, Van Dorpe J, Verhoeven G, Heyns W. Expression of cystatin-related protein and of the C3-component of prostatic-binding protein during postnatal development in the rat ventral prostate and lacrimal gland. Cell Tissue Res 1998;292:115–128.
[105] Winderickx J, Vercaeren I, Verhoeven G, Heyns W. Androgen-dependent expression of cystatin-related protein (CRP) in the exorbital lacrimal gland of the rat. J Steroid Biochem Mol Biol 1994;48:165–170.
[106] Pangerl A, Pangerl B, Jones DJ, Reiter RJ. Beta-adrenoceptors in the extraorbital lacrimal gland of the Syrian hamster. Characterization with [125I]-iodopindolol and evidence of sexual dimorphism. J Neural Transm 1989;77:153–162.
[107] Azzarolo AM, Mazaheri AH, Mircheff AK, Warren DW. Sex-dependent parameters related to electrolyte, water and glycoprotein secretion in rabbit lacrimal glands. Curr Eye Res 1993;12:795–802.
[108] Novak I, Jans IM, Wohlfahrt L. Effect of P2X(7) receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol 2010;588:3615–3627.
[109] Beaucage KL, Xiao A, Pollmann SI, Grol MW, Beach RJ, Holdsworth DW, et al. Loss of P2X7 nucleotide receptor function leads to abnormal fat distribution in mice. Purinergic Signal 2014;10:291–304.
[110] Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in gene expression in human meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52:2727–2740.
[111] Sullivan DA, Rahimi Darabad R, Liu S, Kam WR. Are mice relevant models for understanding sex-related differences in the human meibomian gland? Invest Ophthalmol Vis Sci 2014;55:16.
[112] Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol 2006;124:1286–1292.
[113] Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea 2006;25:651–655.
[114] Satjawatcharaphong P, Ge S, Lin MC. Clinical outcomes associated with thermal pulsation system treatment. Optom Vis Sci 2015;92. e334–41.
[115] Suzuki T. Meibomitis-related keratoconjunctivitis: implications and clinical significance of meibomian gland inflammation. Cornea 2012;31(Suppl 1):S41–S44.
[116] Viso E, Rodriguez-Ares MT, Abelenda D, Oubina B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 2012;53:2601–2606.
[117] Darabad RR, Suzuki T, Richards SM, Jensen RV, Jakobiec FA, Zakka FR, et al. Influence of aromatase absence on the gene expression and histology of the mouse meibomian gland. Invest Ophthalmol Vis Sci 2013;54:987–998.
[118] Liu Y, Knop E, Knop N, Sullivan DA, List EO, Kopchick JJ, et al. Growth hormone influence on the morphology and size of the mouse meibomian gland. J Ophthalmol 2016;2016:5728071.
[119] Elflein HM, Pfeiffer N, Hoffmann EM, Hoehn R, Kottler U, Lorenz K, et al. Correlations between central corneal thickness and general anthropometric characteristics and cardiovascular parameters in a large European cohort from the Gutenberg Health Study. Cornea 2014;33:359–365.
[120] Ito M, Shirakawa R, Arita R, Karasawa Y, Imaki J, Amano S, et al. Observation of whole-mount meibomian glands from cadaveric eyelids using a fructose-based optical clearing agent (abstract). Invest Ophthalmol Vis Sci 2014:55. ARVO E-Abstract 4442.
[121] Ananthi S, Santhosh RS, Nila MV, Prajna NV, Lalitha P, Dharmalingam K. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp Eye Res 2011;92:454–463.
[122] Zheng Y, Huang G, Huang W, He M. Distribution of central and peripheral corneal thickness in Chinese children and adults: the Guangzhou twin eye study. Cornea 2008;27:776–781.
[123] Xu L, Zhang H, Wang YX, Jonas JB. Central corneal thickness and glaucoma in adult Chinese: the Beijing Eye Study. J Glaucoma 2008;17:647–653.
[124] Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma 2014;23:606–612.
[125] Cho P, Lam C. Factors affecting the central corneal thickness of Hong Kong-Chinese. Curr Eye Res 1999;18:368–374.
[126] Kim BJ, Ryu IH, Lee JH, Kim SW. Correlation of sex and myopia with corneal epithelial and stromal thicknesses. Cornea 2016;35:1078–1083.
[127] Lee D-H, Kim D-H, Park S-H. Age and sex related changes in corneal thickness and anterior corneal curvature in Korean young population with Orbscan II topography system. J Opt Soc Korea 2011;15:68–73.
[128] Rushood AA, Zahrani MH, Khamis A, Rushood AA. Central corneal thickness in full-term Saudi newborns. Acta Ophthalmol 2012;90. e355–8.
[129] Vijaya L, George R, Arvind H, Ve Ramesh S, Baskaran M, Raju P, et al. Central corneal thickness in adult south Indians: the Chennai glaucoma study. Ophthalmology 2010;117:700–704.
[130] Henderson JW, Prough WA. Influence of age and sex on flow of tears. Arch Ophthal 1950;43:224–231.
[131] Norn MS. Desiccation of the precorneal film. I. Corneal wetting-time. Acta Ophthalmol (Copenh) 1969;47:865–880.
[132] Lomholt JA, Ehlers N. Graft survival and risk factors of penetrating keratoplasty for microbial keratitis. Acta Ophthalmol Scand 1997;75:418–422.
[133] Allam RS, Khalil NM. Evaluation of sex differences in corneal hysteresis. Eur J Ophthalmol 2015;25:391–395.
[134] Fontes BM, Ambrosio Jr. R, Alonso RS, Jardim D, Velarde GC, Nose W. Corneal biomechanical metrics in eyes with refraction of -19.00 to +9.00 D in healthy Brazilian patients. J Refract Surg 2008;24:941–945.
[135] Narayanaswamy A, Chung RS, Wu RY, Park J, Wong WL, Saw SM, et al. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology 2011;118:1253–1259.
[136] Strobbe E, Cellini M, Barbaresi U, Campos EC. Influence of age and gender on corneal biomechanical properties in a healthy Italian population. Cornea 2014;33:968–972.
[137] Alsbirk PH. Corneal diameter in Greenland Eskimos. Anthropometric and genetic studies with special reference to primary angle-closure glaucoma. Acta Ophthalmol (Copenh) 1975;53:635–646.
[138] Hall LA, Hunt C, Young G, Wolffsohn J. Factors affecting corneoscleral topography. Invest Ophthalmol Vis Sci 2013;54:3691–3701.
[139] Iyamu E, Osuobeni E. Age, gender, corneal diameter, corneal curvature and central corneal thickness in Nigerians with normal intra ocular pressure. J Optom 2012;5:87–97.
[140] Golebiowski B, Papas EB, Stapleton F. Factors affecting corneal and conjunctival sensitivity measurement. Optom Vis Sci 2008;85:241–246.
[141] Acosta MC, Alfaro ML, Borras F, Belmonte C, Gallar J. Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp Eye Res 2006;83:932–938.
[142] Snellingen T, Rao GN, Shrestha JK, Huq F, Cheng H. Quantitative and morphological characteristics of the human corneal endothelium in relation to age, gender, and ethnicity in cataract populations of South Asia. Cornea 2001;20:55–58.
[143] Farjo AA, Halperin GI, Syed N, Sutphin JE, Wagoner MD. Salzmann's nodular corneal degeneration clinical characteristics and surgical outcomes. Cornea 2006;25:11–15.
[144] Graue-Hernandez EO, Mannis MJ, Eliasieh K, Greasby TA, Beckett LA, Bradley JC, et al. Salzmann nodular degeneration. Cornea 2010;29:283–289.
[145] Fernandez-Garcia P, Cervino A, Quiles-Guinau L, Albarran-Diego C, Garcia-Lazaro S, Sanchis-Gimeno JA. Corneal thickness differences between sexes after oxybuprocaine eye drops. Optom Vis Sci 2015;92:89–94.
[146] Fink BA, Sinnott LT, Wagner H, Friedman C, Zadnik K, Group CS. The influence of gender and hormone status on the severity and progression of keratoconus. Cornea 2010;29:65–72.
[147] Naderan M, Shoar S, Kamaleddin MA, Rajabi MT, Naderan M, Khodadadi M. Keratoconus clinical findings according to different classifications. Cornea 2015;34:1005–1011.
[148] Ramdas WD, Vervaet CJ, Bleyen I. Corneal topography for pancorneal toric edge rigid gas-permeable contact lens fitting in patients with keratoconus, and differences in age and gender. Cont Lens Anterior Eye 2014;37:20–25.
[149] Krishnan T, Prajna NV, Gronert K, Oldenburg CE, Ray KJ, Keenan JD, et al. Gender differences in re-epithelialisation time in fungal corneal ulcers. Br J Ophthalmol 2012;96:137–138.
[150] Golas L, Manche EE. Dry eye after laser in situ keratomileusis with femtosecond laser and mechanical keratome. J Cataract Refract Surg 2011;37:1476–1480.
[151] Fanny A, Ouattara A, Aka J, Coulibaly F, Gbe K, Boni S, et al. Ocular biometric values of the black African patient and theoretical consideration of the role of these values in various pathologies: analysis of 325 eyes. J Fr Ophtalmol 2007;30:68–72.
[152] Mehravaran S, Hashemi H, KhabazKhoob M, Fotouhi A. Distribution of radii of curvature of anterior and posterior best fit sphere in a normal population: the Tehran Eye Study. Cont Lens Anterior Eye 2013;36:186–190.
[153] Midelfart A, Aamo B. Ocular parameters in elderly in Norway. Acta Ophthalmol (Copenh) 1994;72:61–66.
[154] Orucoglu F, Akman M, Onal S. Analysis of age, refractive error and gender related changes of the cornea and the anterior segment of the eye with Scheimpflug imaging. Cont Lens Anterior Eye 2015;38:345–350.
[155] Tsai TH, Scheving LE, Scheving LA, Pauly JE. Sex differences in circadian rhythms of several variables in lymphoreticular organs, liver, kidney, and corneal epithelium in adult CD2F1 mice. Anat Rec 1985;211:263–270.
[156] Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J 2012;26:1506–1516.
[157] Gilger BC, Whitley RD, McLaughlin SA, Wright JC, Drane JW. Canine corneal thickness measured by ultrasonic pachymetry. Am J Vet Res 1991;52:1570–1572.
[158] Zurawski CA, McCarey BE, van Rij G, Fernandes A. Corneal biometrics of the rhesus monkey (Macaca mulatta). J Med Primatol 1989;18:461–466.
[159] Connor CG, Flockencier LL, Hall CW. The influence of gender on the ocular surface. J Am Optom Assoc 1999;70:182–186.
[160] Baig R, Ali AW, Ali T, Ali A, Shah MN, Sarfaraz A, et al. Prevalence of allergic conjunctivitis in school children of Karachi. J Pak Med Assoc 2010;60:371–373.
[161] Gelardi M, Leo ME, Quaranta VN, Iannuzzi L, Tripodi S, Quaranta N, et al. Clinical characteristics associated with conjunctival inflammation in allergic rhinoconjunctivitis. J Allergy Clin Immunol Pract 2015;3:387–391. e1.
[162] Droutsas K, Sekundo W. Epidemiology of pterygium. A review. Ophthalmologe 2010;107:511–512. 4-6.
[163] Wilson G, Horner D, Begley C, Page J. Ocular discomfort from pterygium in men and women. Eye Contact Lens 2008;34:201–206.
[164] Mimura T, Usui T, Obata H, Yamagami S, Mori M, Funatsu H, et al. Severity and determinants of pinguecula in a hospital-based population. Eye Contact Lens 2011;37:31–35.
[165] Theodore FH. Superior limbic keratoconjunctivitis. Eye Ear Nose Throat Mon 1963;42:25–28.
[166] Mendoza-Adam GR-GA. Superior limbic keratoconjunctivitis (SLK) and its association to systemic diseases. Rev Mex Oft 2013;87:93–99.
[167] Perry LJ, Jakobiec FA, Zakka FR. Bacterial and mucopeptide concretions of the lacrimal drainage system: an analysis of 30 cases. Ophthal Plast Reconstr Surg 2012;28:126–133.
[168] Ramey NA, Hoang JK, Richard MJ. Multidetector CT of nasolacrimal canal morphology: normal variation by age, gender, and race. Ophthal Plast Reconstr Surg 2013;29:475–480.
[169] Takahashi Y, Kakizaki H, Nakano T. Bony nasolacrimal duct entrance diameter: gender difference in cadaveric study. Ophthal Plast Reconstr Surg 2011;27:204–205.
[170] McCormick A, Sloan B. The diameter of the nasolacrimal canal measured by computed tomography: gender and racial differences. Clin Exp Ophthalmol 2009;37:357–361.
[171] Groessl SA, Sires BS, Lemke BN. An anatomical basis for primary acquired nasolacrimal duct obstruction. Arch Ophthalmol 1997;115:71–74.
[172] Czyz CN, Bacon TS, Stacey AW, Cahill EN, Costin BR, Karanfilov BI, et al. Nasolacrimal system aeration on computed tomographic imaging: sex and age variation. Ophthal Plast Reconstr Surg 2016;32:11–16.
[173] Shigeta K, Takegoshi H, Kikuchi S. Sex and age differences in the bony nasolacrimal canal: an anatomical study. Arch Ophthalmol 2007;125:1677–1681.
[174] Yamamoto Y, Yokoi N, Higashihara H, Inagaki K, Sonomura Y, Komuro A, et al. Clinical characteristics of short tear film breakup time (BUT) -type dry eye. Nippon Ganka Gakkai Zasshi 2012;116:1137–1143.
[175] Maissa C, Guillon M. Tear film dynamics and lipid layer characteristics–effect of age and gender. Cont Lens Anterior Eye 2010;33:176–182.
[176] van Setten GB, Schultz GS, Macauley S. Growth factors in human tear fluid and in lacrimal glands. Adv Exp Med Biol 1994;350:315–319.
[177] Nava A, Barton K, Monroy DC, Pflugfelder SC. The effects of age, gender, and fluid dynamics on the concentration of tear film epidermal growth factor. Cornea 1997;16:430–438.
[178] Barton K, Nava A, Monroy DC, Pflugfelder SC. Cytokines and tear function in ocular surface disease. Adv Exp Med Biol 1998;438:461–469.
[179] Cho P, Yap M. Age, gender, and tear break-up time. Optom Vis Sci 1993;70:828–831.
[180] Craig JP, Tomlinson A. Effect of age on tear osmolality. Optom Vis Sci 1995;72:713–717.
[181] Craig JP, Tomlinson A. Age and gender effects on the normal tear film. Adv Exp Med Biol 1998;438:411–415.
[182] Lim RR, Tan A, Liu YC, Barathi VA, Mohan RR, Mehta JS, et al. ITF2357 transactivates Id3 and regulate TGFbeta/BMP7 signaling pathways to attenuate corneal fibrosis. Sci Rep 2016;6:20841.
[183] Snyder C, Fullard RJ. Clinical profiles of non dry eye patients and correlations with tear protein levels. Int Ophthalmol 1991;15:383–389.
[184] Marcozzi G, Liberati V, Madia F, Centofanti M, de Feo G. Age- and gender-related differences in human lacrimal fluid peroxidase activity. Ophthalmologica 2003;217:294–297.
[185] Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol 2002;506:989–998.
[186] du Toit R. Situ P, Simpson T, Fonn D. The effects of six months of contact lens wear on the tear film, ocular surfaces, and symptoms of presbyopes. Optom Vis Sci 2001;78:455–462.
[187] Albietz JM, Lenton LM, McLennan SG. Effect of laser in situ keratomileusis for hyperopia on tear film and ocular surface. J Refract Surg 2002;18:113–123.
[188] Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 2003;110:1096–1101.
[189] Han SB, Yang HK, Hyon JY, Hwang JM. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefes Arch Clin Exp Ophthalmol 2013;251:791–796.
[190] Verhagen C, Rowshani T, Willekens B, van Haeringen NJ. Spontaneous development of corneal crystalline deposits in MRL/Mp mice. Invest Ophthalmol Vis Sci 1995;36:454–461.
[191] Vujkovic V, Mikac G, Kozomara R. Distribution and density of conjunctival goblet cells. Med Pregl 2002;55:195–200.
[192] Ueno H, Ariji E, Izumi M, Uetani M, Hayashi K, Nakamura T. MR imaging of the lacrimal gland. Age-related and gender-dependent changes in size and structure. Acta Radiol 1996;37:714–719.
[193] Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology 1995;102:678–686.
[194] Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea 2006;25:S82–S89.
[195] Waterhouse JP. Focal adenitis in salivary and lacrimal glands. Proc R Soc Med 1963;56:911–918.
[196] Tang SX, Lim RP, Al-Dahmash S, Blaydon SM, Cho RI, Choe CH, et al. Bilateral lacrimal gland disease: clinical features of 97 cases. Ophthalmology 2014;121:2040–2046.
[197] Bukhari AA, Basheer NA, Joharjy HI. Age, gender, and interracial variability of normal lacrimal gland volume using MRI. Ophthal Plast Reconstr Surg 2014;30:388–391.
[198] Lorber M. Gross characteristics of normal human lacrimal glands. Ocul Surf 2007;5:13–22.
[199] Hahn JD. Effect of cyproterone acetate on sexual dimorphism of the exorbital lacrimal gland in rats. J Endocrinol 1969;45:421–424.
[200] Kelly RB, Oliver C, Hand AR. The effects of vinblastine on acinar cells of the exorbital lacrimal gland of the rat. Cell Tissue Res 1978;195:227–237.
[201] Luciano L. The fine structure of the tear glands of the rat and their sex dimorphism. Z Zellforsch Mikrosk Anat 1967;76:1–20.
[202] Paulini K, Mohr W, Beneke G, Kulka R. Age- und sex-dependent changes in glandular cells. II. Cytophotometric and autoradiographic investigations on the glandula lacrimalis, glandula infraorbitalis and glandula orbitalis externa of the rat. Gerontologia 1972;18:147–156.
[203] Ricciardi MP, Gesi M, Giannessi E, Soldani P, Stornelli MR, Lenzi P. Comparative analysis of senescent exorbital lacrimal glands in male and female albino rats. J Submicrosc Cytol Pathol 2002;34:167–175.
[204] Sullivan DA, Block L, Pena JD. Influence of androgens and pituitary hormones on the structural profile and secretory activity of the lacrimal gland. Acta Ophthalmol Scand 1996;74:421–435.
[205] Sullivan DA, Hann LE, Yee L, Allansmith MR. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol (Copenh) 1990;68:188–194.
[206] Saunier B, Triyatni M, Ulianich L, Maruvada P, Yen P, Kohn LD. Role of the asialoglycoprotein receptor in binding and entry of hepatitis C virus structural proteins in cultured human hepatocytes. J Virol 2003;77:546–559.
[207] De Vita S, Damato R, De Marchi G, Sacco S, Ferraccioli G. True primary Sjögren's syndrome in a subset of patients with hepatitis C infection: a model linking chronic infection to chronic sialadenitis. Isr Med Assoc J 2002;4:1101–1105.
[208] Toussirot E, Le Huede G, Mougin C, Balblanc JC, Bettinger D, Wendling D. Presence of hepatitis C virus RNA in the salivary glands of patients with Sjögren's syndrome and hepatitis C virus infection. J Rheumatol 2002;29:2382–2385.
[209] Loustaud-Ratti V, Riche A, Liozon E, Labrousse F, Soria P, Rogez S, et al. Prevalence and characteristics of Sjögren's syndrome or Sicca syndrome in chronic hepatitis C virus infection: a prospective study. J Rheumatol 2001;28:2245–2251.
[210] Ramos-Casals M, Garcia-Carrasco M, Brito Zeron MP, Cervera R, Font J. Viral etiopathogenesis of Sjögren's syndrome: role of the hepatitis C virus. Autoimmun Rev 2002;1:238–243.
[211] Siagris D, Pharmakakis N, Christofidou M, Petropoulos JK, Vantzou C, Lekkou A, et al. Keratoconjunctivitis sicca and chronic HCV infection. Infection 2002;30:229–233.
[212] Villalta D, Mytilinaiou MG, Elsner M, Hentschel C, Cuccato J, Somma V, et al. Autoantibodies to asialoglycoprotein receptor (ASGPR) in patients with autoimmune liver diseases. Clin Chim Acta 2015;450:1–5.
[213] Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev 1989;69:383–416.
[214] Guttridge NM. Changes in ocular and visual variables during the menstrual cycle. Ophthalmic Physiol Opt 1994;14:38–48.
[215] Imafidon C, Akingbade A, Imafidon J, Onwudiegwu U. Anterior segment adaptations in gestation. Optom Today April 5, 1993.
[216] Imafidon CO, Imafidon JE. Contact lenses in pregnancy. Br J Obstet Gynaecol 1992;99:865–867.
[217] Leach NE, Wallis NE, Lothringer LL, Olson JA. Corneal hydration changes during the normal menstrual cycle–a preliminary study. J Reprod Med 1971;6:201–204.
[218] Midelfart A. Women and men–same eyes? Acta Ophthalmol Scand 1996;74:589–592.
[219] Millodot M, Lamont A. Influence of menstruation on corneal sensitivity. Br J Ophthalmol 1974;58:752–756.
[220] Riss B, Binder S, Riss P, Kemeter P. Corneal sensitivity during the menstrual cycle. Br J Ophthalmol 1982;66:123–126.
[221] Serrander AM, Peek KE. Changes in contact lens comfort related to the menstrual cycle and menopause. A review of articles. J Am Optom Assoc 1993;64:162–166.
[222] Soni PS. Effects of oral contraceptive steroids on the thickness of human cornea. Am J Optom Physiol Opt 1980;57:825–834.
[223] Chen Z, Tong L, Li Z, Yoon KC, Qi H, Farley W, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci 2008;49:539–549.
[224] Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, Kinoshita S. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci 2001;42:549–556.
[225] Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008;8:737–744.
[226] Ghazeeri G, Abdullah L, Abbas O. Immunological differences in women compared with men: overview and contributing factors. Am J Reprod Immunol 2011;66:163–169.
[227] Bouman A, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol 2004;52:19–26.
[228] Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol 2001;81:254–262.
[229] Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009;15:955–959.
[230] Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 2006;177:2088–2096.
[231] Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1995;1:1279–1283.
[232] Rowley MJ, Mackay IR. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol 1969;5:407–418.
[233] Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science 1999;283:1277–1278.
[234] Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 2007;178:2572–2578.
[235] Abramowitz LK, Olivier-Van Stichelen S, Hanover JA. Chromosome imbalance as a driver of sex disparity in disease. J Genomics 2014;2:77–88.
[236] Kocar IH, Yesilova Z, Ozata M, Turan M, Sengul A, Ozdemir I. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter's syndrome. Clin Exp Immunol 2000;121:448–452.
[237] Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun 2012;38:J282–J291.
[238] Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 2011;33:791–802.
[239] Dai R, Ahmed SA. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Ther Clin Risk Manag 2014;10:151–163.
[240] Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, et al. Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun 2013;41:60–71.
[241] Tomkovich S, Jobin C. Microbiota and host immune responses: a love-hate relationship. Immunology 2016;147:1–10.
[242] Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014;35:97–104.
[243] Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013;339:1084–1088.
[244] Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–412.
[245] Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 2012;11:A479–A485.
[246] McDermott AM. Defence mechanisms of tears and ocular surface. In: Besharse J, Dana R, editors. Encyclopedia of the Eye, vol. 1. Cambridge, MA: Academic Press; 2010. p. 1–8.
[247] Mircheff AK. Adaptive immune system and the eye: mucosal immunity. In: Besharse J, Dana R, editors. Encyclopedia of the Eye, vol. 1. Cambridge, MA: Academic Press; 2010. p. 33–40.
[248] McDermott AM. Antimicrobial compounds in tears. Exp Eye Res 2013;117:53–61.
[249] Sen DK, Sarin GS, Mathur GP, Saha K. Biological variation of immunoglobulin concentrations in normal human tears related to age and sex. Acta Ophthalmol (Copenh) 1978;56:439–444.
[250] Tchah H. Measurement of IgA level in normal human tears by enzyme-linked immunosorbent assay. Korean J Ophthalmol 1989;3:70–74.
[251] Mii S, Nakamura K, Takeo K, Kurimoto S. Analysis of human tear proteins by two-dimensional electrophoresis. Electrophoresis 1992;13:379–382.
[252] Balasubramanian SA, Pye DC, Willcox MD. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp Eye Res 2012;96:132–137.
[253] Kijlstra A, Jeurissen SH, Koning KM. Lactoferrin levels in normal human tears. Br J Ophthalmol 1983;67:199–202.
[254] Jensen OL, Gluud BS, Birgens HS. The concentration of lactoferrin in tears of normals and of diabetics. Acta Ophthalmol (Copenh) 1986;64:83–87.
[255] Aine E, Morsky P. Lysozyme concentration in tears–assessment of reference values in normal subjects. Acta Ophthalmol (Copenh) 1984;62:932–938.
[256] Saari KM, Aho V, Paavilainen V, Nevalainen TJ. Group II PLA(2) content of tears in normal subjects. Invest Ophthalmol Vis Sci 2001;42:318–320.
[257] Sindt CW, Grout TK, Critser DB, Kern JR, Meadows DL. Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study. Clin Ophthalmol 2012;6:511–519.
[258] O'Brien RL, Taylor MA, Hartley J, Nuhsbaum T, Dugan S, Lahmers K, et al. Protective role of gammadelta T cells in spontaneous ocular inflammation. Invest Ophthalmol Vis Sci 2009;50:3266–3274.
[259] Gudmundsson OG, Bjornsson J, Olafsdottir K, Bloch KJ, Allansmith MR, Sullivan DA. T cell populations in the lacrimal gland during aging. Acta Ophthalmol (Copenh) 1988;66:490–497.
[260] Nasu M, Matsubara O, Yamamoto H. Post-mortem prevalence of lymphocytic infiltration of the lacrymal gland: a comparative study in autoimmune and non-autoimmune diseases. J Pathol 1984;143:11–15.
[261] Morgan PB, Efron N, Brennan NA, Hill EA, Raynor MK, Tullo AB. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Invest Ophthalmol Vis Sci 2005;46:3136–3143.
[262] Dart JK, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology 2008;115(1647–54):e1–3. 54.
[263] Nguyen C, Singson E, Kim JY, Cornelius JG, Attia R, Doyle ME, et al. Sjögren's syndrome-like disease of C57BL/6.NOD-Aec1 Aec2 mice: gender differences in keratoconjunctivitis sicca defined by a cross-over in the chromosome 3 Aec1 locus. Scand J Immunol 2006;64:295–307.
[264] Lieberman SM, Kreiger PA, Koretzky GA. Reversible lacrimal gland-protective regulatory T-cell dysfunction underlies male-specific autoimmune dacryoadenitis in the non-obese diabetic mouse model of Sjögren syndrome. Immunology 2015;145:232–241.
[265] Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol 2015;195:3086–3099.
[266] McCaffery M. Pasero C Assessment: underlying complexities, misconceptions, and practical tools. In: McCaffery M, Pasero C, editors. Pain Clinical Manual. St. Louis, MO: Mosby Inc.; 1999. p. 35–102.
[267] Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain 2008;137:473–477.
[268] Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II Pain and Sensation report. Ocul Surf 2017;15:404–437.
[269] Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15:241–249.
[270] van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth 2013;111:13–18.
[271] Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333.
[272] Walid MS, Donahue SN, Darmohray DM, Hyer LA, Robinson JS. The fifth vital sign–what does it mean? Pain Pract 2008;8:417–422.
[273] Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29:301–312.
[274] Galor A, Zlotcavitch L, Walter SD, Felix ER, Feuer W, Martin ER, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol 2015;99:665–668.
[275] Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med 2005;2:137–145.
[276] Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132(Suppl 1):S26–S45.
[277] Craft RM. Modulation of pain by estrogens. Pain 2007;132(Suppl 1):S3–S12.
[278] Bandeen-Roche K, Munoz B, Tielsch JM, West SK, Schein OD. Self-reported assessment of dry eye in a population-based setting. Invest Ophthalmol Vis Sci 1997;38:2469–2475.
[279] Begley CG, Chalmers RL, Mitchell GL, Nichols KK, Caffery B, Simpson T, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea 2001;20:610–618.
[280] Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye 2010;33:55–60.
[281] Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016;100:128–134.
[282] Galor A, Covington D, Levitt AE, McManus KT, Seiden B, Felix ER, et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain 2015;17:310–318.
[283] Türkyilmaz K, Türkyilmaz AK, Kurt EE, Kurt A, Öner V. Dry eye in patients with fibromyalgia and its relevance to functional and emotional status. Cornea 2013;32:862–866.
[284] Chen CH, Yang TY, Lin CL, Chen CS, Lin WM, Kuo CN, et al. Dry eye syndrome risks in patients with fibromyalgia: a national retrospective cohort study. Medicine (Baltimore) 2016;95:e2607.
[285] Berry PH, Chapman C, Covington EC, Dahl JL, Katz JA, et al. Pain: Current understanding of assessment, management, and treatments. Preston White Drive Reston, VA: National Pharmaceutical Council, Inc.; 2001.
[286] Schaumberg DA, Gulati A, Mathers WD, Clinch T, Lemp MA, Nelson JD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf 2007;5:50–57.
[287] Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol 1999;83:390–395.
[288] Segal BM, Pogatchnik B, Holker E, Liu H, Sloan J, Rhodus N, et al. Primary Sjögren's syndrome: cognitive symptoms, mood, and cognitive performance. Acta Neurol Scand 2012;125:272–278.
[289] Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res 2004;78:513–525.
[290] Bourcier T, Acosta MC, Borderie V, Borrás F, Gallar J, Bury T, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci 2005;46:2341–2345.
[291] De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol 2004;137:109–115.
[292] Golebiowski B, Papas EB, Stapleton F. Corneal and conjunctival sensory function: the impact on ocular surface sensitivity of change from low to high oxygen transmissibility contact lenses. Invest Ophthalmol Vis Sci 2012;53:1177–1181.
[293] Vehof J, Kozareva D, Hysi PG, Harris J, Nessa A, Williams FK, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol 2013;131:1304–1308.
[294] Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, Nibourg SA, Hammond CJ. Clinical characteristics of dry eye patients with chronic pain syndromes. Am J Ophthalmol 2016;166:203–204.
[295] Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev 2014;39:61–78.
[296] Racine M, Dion D, Dupuis G, Guerriere DN, Zagorski B, Choinière M, et al. The Canadian STOP-PAIN project: the burden of chronic pain-does sex really matter? Clin J Pain 2014;30:443–452.
[297] Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–485.
[298] Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–58.
[299] Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med 2009;10:289–299.
[300] Barnabe C, Bessette L, Flanagan C, Leclercq S, Steiman A, Kalache F, et al. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol 2012;39:1221–1230.
[301] Gerdle B, Björk J, Cöster L, Henriksson K, Henriksson C, Bengtsson A. Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord 2008;9:102.
[302] Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain 2012;153:602–618.
[303] Moore QL, De Paiva CS, Pflugfelder SC. Effects of dry eye therapies on environmentally induced ocular surface disease. Am J Ophthalmol 2015;160. 135–42.e1.
[304] Teson M, Gonzalez-Garcia MJ, Lopez-Miguel A, Enriquez-de-Salamanca A, Martin-Montanez V, Benito MJ, et al. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Invest Ophthalmol Vis Sci 2013;54:2093–2099.
[305] Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the study of ocular surface disease. Ocul Surf 2005;3:143–154.
[306] Chao C, Stapleton F, Badarudin E, Golebiowski B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom Vis Sci 2015;92:183–189.
[307] Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci 1999;40:513–519.
[308] Tesón M, Calonge M, Fernández I, Stern ME, González-García MJ. Characterization by Belmonte's gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Invest Ophthalmol Vis Sci 2012;53:3154–3160.
[309] Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, et al. The TFOS international workshop on contact lens discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci 2013;54:TFOS71–97.
[310] Khezri F, Mirzajani A, Karimian F, Jafarzadehpur E. Is corneal sensitivity sex dependent? J Ophthalmic Vis Res 2015;10:102–105.
[311] Versura P, Giannaccare G, Fresina M, Campos EC. Subjective discomfort symptoms are related to low corneal temperature in patients with evaporative dry eye. Cornea 2015;34:1079–1085.
[312] Cavdar E, Ozkaya A, Alkin Z, Ozkaya HM, Babayigit MA. Changes in tear film, corneal topography, and refractive status in premenopausal women during menstrual cycle. Cont Lens Anterior Eye 2014;37:209–212.
[313] Versura P, Fresina M, Campos EC. Ocular surface changes over the menstrual cycle in women with and without dry eye. Gynecol Endocrinol 2007;23:385–390.
[314] Scuderi G, Contestabile MT, Gagliano C, Iacovello D, Scuderi L, Avitabile T. Effects of phytoestrogen supplementation in postmenopausal women with dry eye syndrome: a randomized clinical trial. Can J Ophthalmol 2012;47:489–492.
[315] Forsblad-d'Elia H, Carlsten H, Labrie F, Konttinen YT, Ohlsson C. Low serum levels of sex steroids are associated with disease characteristics in primary Sjögren's syndrome; supplementation with dehydroepiandrosterone restores the concentrations. J Clin Endocrinol Metab 2009;94:2044–2051.
[316] Azcarate PM, Venincasa VD, Feuer W, Stanczyk F, Schally AV, Galor A. Androgen deficiency and dry eye syndrome in the aging male. Invest Ophthalmol Vis Sci 2014;55:5046–5053.
[317] Mogil JS. Pain genetics: past, present and future. Trends Genet 2012;28:258–266.
[318] Na KS, Mok JW, Kim JY, Joo CK. Proinflammatory gene polymorphisms are potentially associated with Korean non-Sjögren dry eye patients. Mol Vis 2011;17:2818–2823.
[319] Vehof J, Wang B, Kozareva D, Hysi PG, Snieder H, Hammond CJ. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci 2014;55:7278–7283.
[320] Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast 2015;2015:504691.
[321] Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014;66:80–101.
[322] Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–3105.
[323] Sharp TJ. The prevalence of post-traumatic stress disorder in chronic pain patients. Curr Pain Headache Rep 2004;8:111–115.
[324] Modi YS, Qurban Q, Zlotcavitch L, Echeverri RJ, Feuer W, Florez H, et al. Ocular surface symptoms in veterans returning from operation Iraqi freedom and operation enduring freedom. Invest Ophthalmol Vis Sci 2014;55:650–653.
[325] Fernandez CA, Galor A, Arheart KL, Musselman DL, Venincasa VD, Florez HJ, et al. Dry eye dyndrome, posttraumatic stress disorder, and depression in an older male veteran population. Invest Ophthalmol Vis Sci 2013;54:3666–3672.
[326] Galor A, Feuer W, Lee DJ, Florez H, Faler AL, Zann KL, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol 2012;154:340–346. e2.
[327] Galor A, Felix ER, Feuer W, Shalabi N, Martin ER, Margolis TP, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol 2015;99:1126–1129.
[328] Na KS, Han K, Park YG, Na C, Joo CK. Depression, stress, quality of life, and dry eye disease in Korean women: a population-based study. Cornea 2015;34:733–738.
[329] Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res 2015:1–6.
[330] Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat 2015;11:889–894.
[331] Hallak JA, Tibrewal S, Jain S. Depressive symptoms in patients with dry eye disease: a case-control study using the Beck Depression Inventory. Cornea 2015;34:1545–1550.
[332] Kim KW, Han SB, Han ER, Woo SJ, Lee JJ, Yoon JC, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci 2011;52:7954–7958.
[333] Labbé A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol 2013;97:1399–1403.
[334] Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res 2011;36:1–7.
[335] Liyue H, Chiang PP, Sung SC, Tong L. Dry eye-related visual blurring and irritative symptoms and their association with depression and anxiety in eye clinic patients. Curr Eye Res 2015:1–10.
[336] van der Vaart R, Weaver MA, Lefebvre C, Davis RM. The association between dry eye disease and depression and anxiety in a large population-based study. Am J Ophthalmol 2015;159:470–474.
[337] Mattei D, Schaefer CE. An investigation of validity of the subjective happiness scale. Psychol Rep 2004;94:288–290.
[338] Kawashima M, Uchino M, Yokoi N, Uchino Y, Dogru M, Komuro A, et al. Associations between subjective happiness and dry eye disease: a new perspective from the Osaka study. PLoS One 2015;10:e0123299.
[339] Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17:52–64.
[340] Keogh E, Denford S. Sex differences in perceptions of pain coping strategy usage. Eur J Pain 2009;13:629–634.
[341] Segal BM, Pogatchnik B, Rhodus N, Sivils KM, McElvain G, Solid CA. Pain in primary Sjögren's syndrome: the role of catastrophizing and negative illness perceptions. Scand J Rheumatol 2014;43:234–241.
[342] Oswald PA. An examination of the current usefulness of the Bem Sex-Role Inventory. Psychol Rep 2004;94:1331–1336.
[343] Alabas OA, Tashani OA, Tabasam G, Johnson MI. Gender role affects experimental pain responses: a systematic review with meta-analysis. Eur J Pain 2012;16:1211–1223.
[344] Kállai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain 2004;112:142–147.
[345] Alabas OA, Tashani OA, Johnson MI. Effects of ethnicity and gender role expectations of pain on experimental pain: a cross-cultural study. Eur J Pain 2013;17:776–786.
[346] Rahim-Williams B, Riley JL, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med 2012;13:522–540.
[347] Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012;153:619–635.
[348] Sullivan DA, Wickham LA, Krenzer KL, Rocha EM, Toda I. Aqueous tear deficiency in Sjögren’s syndrome: Possible causes and potential treatment. In: Pleyer U, Hartmann C, Sterry W, editors. Oculodermal Diseases - Immunology of Bullous Oculo-Muco-Cutaneous Disorders. Buren, The Netherlands: Aeolus Press; 1997. p. 95–152.
[349] Versura P, Giannaccare G, Campos EC. Sex-steroid imbalance in females and dry eye. Curr Eye Res 2015;40:162–175.
[350] Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom 2014;97:324–336.
[351] Richards SM, Liu M, Jensen RV, Schirra F, Yamagami H, Lombardi MJ, et al. Androgen regulation of gene expression in the mouse lacrimal gland. J Steroid Biochem Mol Biol 2005;96:401–413.
[352] Suzuki T, Schirra F, Richards SM, Treister NS, Lombardi MJ, Rowley P, et al. Estrogen's and progesterone's impact on gene expression in the mouse lacrimal gland. Invest Ophthalmol Vis Sci 2006;47:158–168.
[353] Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci 1999;40:1261–1265.
[354] Sharma A, Hindman HB. Aging: a predisposition to dry eyes. J Ophthalmol 2014;2014:781683.
[355] Gagliano C, Caruso S, Napolitano G, Malaguarnera G, Cicinelli MV, Amato R, et al. Low levels of 17-β-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: a case–control study. Br J Ophthalmol 2014;98:371–376.
[356] Oprea L, Tiberghien A, Creuzot-Garcher C, Baudouin C. Hormonal regulatory influence in tear film]. J Fr Ophtalmol 2004;27:933–941.
[357] Vehof J, Hysi PG, Hammond CJ. A metabolome-wide study of dry eye disease (abstract). Invest Ophthalmol Vis Sci 2014:55. ARVO E-Abstract 3054.
[358] Ariga H, Edwards J, Sullivan DA. Androgen control of autoimmune expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Clin Immunol Immunopathol 1989;53:499–508.
[359] Aumuller G, Arce EA, Heyns W, Vercaeren I, Dammshauser I, Seitz J. Immunocytochemical localization of seminal proteins in salivary and lacrimal glands of the rat. Cell Tissue Res 1995;280:171–181.
[360] Azzarolo AM, Bjerrum K, Maves CA, Becker L, Wood RL, Mircheff AK, et al. Hypophysectomy-induced regression of female rat lacrimal glands: partial restoration and maintenance by dihydrotestosterone and prolactin. Invest Ophthalmol Vis Sci 1995;36:216–226.
[361] Azzarolo AM, Mircheff AK, Kaswan RL, Stanczyk FZ, Gentschein E, Becker L, et al. Androgen support of lacrimal gland function. Endocrine 1997;6:39–45.
[362] Azzarolo AM, Wood RL, Mircheff AK, Richters A, Olsen E, Berkowitz M, et al. Androgen influence on lacrimal gland apoptosis, necrosis, and lymphocytic infiltration. Invest Ophthalmol Vis Sci 1999;40:592–602.
[363] Claessens F, Vanaken H, Vercaeren I, Verrijdt G, Haelens A, Schoenmakers E, et al. Androgen-regulated transcription in the epithelium of the rat lacrimal gland. Adv Exp Med Biol 1998;438:43–48.
[364] Hann LE, Kelleher RS, Sullivan DA. Influence of culture conditions on the androgen control of secretory component production by acinar cells from the rat lacrimal gland. Invest Ophthalmol Vis Sci 1991;32:2610–2621.
[365] Kao WW-Y, Wang I-J, Spaulding AG, Fundergurgh J, Liu C-Y. Overexpression of Biglycan Induces Ocular Surface Disorders in Ktcnpr-Bgln Transgenic Mice (abstract). Third Int Conf Lacrimal Gl Tear Film Dry Eye Syndromes Basic Sci Clin Relevance 2000;19(Supplement 2):S98.
[366] Kelleher RS, Hann LE, Edwards JA, Sullivan DA. Endocrine, neural, and immune control of secretory component output by lacrimal gland acinar cells. J Immunol 1991;146:3405–3412.
[367] Lambert RW, Kelleher RS, Wickham LA, Vaerman JP, Sullivan DA. Neuroendocrinimmune modulation of secretory component production by rat lacrimal, salivary, and intestinal epithelial cells. Invest Ophthalmol Vis Sci 1994;35:1192–1201.
[368] Liu M, Richards SM, Schirra F, Yamagami H, Sullivan BD, Sullivan DA. Identification of androgen-regulated genes in the lacrimal gland. Adv Exp Med Biol 2002;506:129–135.
[369] Luo F, Zhang H, Sun X. The change of tear secretion and tear film stability in castrated male rabbits. Zhonghua Yan Ke Za Zhi 2001;37:458–461.
[370] Myal Y, Iwasiow B, Cosby H, Yarmill A, Blanchard A, Tsuyuki D, et al. Analysis of tissue- and hormone-specific regulation of the human prolactin-inducible protein/gross cystic disease fluid protein-15 gene in transgenic mice. J Mol Endocrinol 1998;21:217–223.
[371] Myal Y, Iwasiow B, Yarmill A, Harrison E, Paterson JA, Shiu RP. Tissue-specific androgen-inhibited gene expression of a submaxillary gland protein, a rodent homolog of the human prolactin-inducible protein/GCDFP-15 gene. Endocrinology 1994;135:1605–1610.
[372] Ono M, Rocha FJ, Sullivan DA. Immunocytochemical location and hormonal control of androgen receptors in lacrimal tissues of the female MRL/Mp-lpr/lpr mouse model of Sjögren's syndrome. Exp Eye Res 1995;61:659–666.
[373] Rocha EM, Wickham LA, Huang Z, Toda I, Gao J, da Silveira LA, et al. Presence and testosterone influence on the levels of anti- and pro-inflammatory cytokines in lacrimal tissues of a mouse model of Sjögren's syndrome. Adv Exp Med Biol 1998;438:485–491.
[374] Rocha F, Sato E, Sullivan B, Sullivan D. Effect of androgen analogue treatment and androgen withdrawal on lacrimal gland inflammation in a mouse model (MRL/Mp-lpr/lpr) of Sjögren’s syndrome. Reg Immunol 1994;6:270–277.
[375] Sato EH, Ariga H, Sullivan DA. Impact of androgen therapy in Sjögren's syndrome: hormonal influence on lymphocyte populations and Ia expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Invest Ophthalmol Vis Sci 1992;33:2537–2545.
[376] Sato E, Sullivan D. Comparative influence of steroid hormones and immunosuppressive agents on autoimmune expression in lacrimal glands of a female mouse model of Sjögren's syndrome. Invest Ophthalmol Vis Sci 1994;35:2632–2642.
[377] Sullivan DA, Edwards JA. Androgen stimulation of lacrimal gland function in mouse models of Sjögren's syndrome. J Steroid Biochem Mol Biol 1997;60:237–245.
[378] Sullivan DA, Kelleher RS, Vaerman JP, Hann LE. Androgen regulation of secretory component synthesis by lacrimal gland acinar cells in vitro. J Immunol 1990;145:4238–4244.
[379] Sullivan DA, Sato EH. Immunology of the lacrimal gland. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology: Basic Sciences. Philadelphia, PA: WB Saunders Company; 1994. p. 479–486.
[380] Toda I, Sullivan BD, Wickham LA, Sullivan DA. Gender- and androgen-related influence on the expression of proto-oncogene and apoptotic factor mRNAs in lacrimal glands of autoimmune and non-autoimmune mice. J Steroid Biochem Mol Biol 1999;71:49–61.
[381] Vanaken H, Claessens F, Vercaeren I, Heyns W, Peeters B, Rombauts W. Androgenic induction of cystatin-related protein and the C3 component of prostatic binding protein in primary cultures from the rat lacrimal gland. Mol Cell Endocrinol 1996;121:197–205.
[382] Vanaken H, Gerard RD, Verrijdt G, Haelens A, Rombauts W, Claessens F. Tissue-specific androgen responses in primary cultures of lacrimal epithelial cells studied by adenoviral gene transfer. J Steroid Biochem Mol Biol 2001;78:319–328.
[383] Vanaken H, Vercaeren I, Claessens F, De Vos R, Dewolf-Peeters C, Vaerman JP, et al. Primary rat lacrimal cells undergo acinar-like morphogenesis on reconstituted basement membrane and express secretory component under androgen stimulation. Exp Cell Res 1998;238:377–388.
[384] Vendramini AC, Soo C, Sullivan DA. Testosterone-induced suppression of autoimmune disease in lacrimal tissue of a mouse model (NZB/NZW F1) of Sjögren's syndrome. Invest Ophthalmol Vis Sci 1991;32:3002–3006.
[385] Vercaeren I, Vanaken H, Devos A, Peeters B, Verhoeven G, Heyns W. Androgens transcriptionally regulate the expression of cystatin-related protein and the C3 component of prostatic binding protein in rat ventral prostate and lacrimal gland. Endocrinology 1996;137:4713–4720.
[386] Vercaeren I, Winderickx J, Devos A, Peeters B, Heyns W. An effect of androgens on the length of the poly(A)-tail and alternative splicing cause size heterogeneity of the messenger ribonucleic acids encoding cystatin-related protein. Endocrinology 1993;132:2496–2502.
[387] Warren DW, Azzarolo AM, Huang ZM, Platler BW, Kaswan RL, Gentschein E, et al. Androgen support of lacrimal gland function in the female rabbit. Adv Exp Med Biol 1998;438:89–93.
[388] Wickham LA, Huang Z, Lambert RW, Sullivan DA. Effect of sialodacryoadenitis virus exposure on acinar epithelial cells from the rat lacrimal gland. Ocul Immunol Inflamm 1997;5:181–195.
[389] Wickham LA, Rocha EM, Gao J, Krenzer KL, da Silveira LA, Toda I, et al. Identification and hormonal control of sex steroid receptors in the eye. Adv Exp Med Biol 1998;438:95–100.
[390] Cavallero C, Ofner P. Relative effectiveness of various steroids in an androgen assay using the exorbital lacrimal gland of the castrated rat. II. C 19-steroids of the 5-alpha-androstane series. Acta Endocrinol (Copenh) 1967;55:131–135.
[391] Sullivan DA, Belanger A, Cermak JM, Berube R, Papas AS, Sullivan RM, et al. Are women with Sjögren's syndrome androgen-deficient? J Rheumatol 2003;30:2413–2419.
[392] Sullivan DA, Schaumberg DA, Schirra F, Suzuki T, Liu M, Richards SM, et al. Sex, sex steroids and dry eye syndromes. In: Zierhut M, Stern ME, Sullivan DA, editors. Immunology of the Lacrimal Gland, Tear Film and Ocular Surface. London: Taylor & Francis; 2005. p. 161–181.
[393] Li L, Kang Q, Wang S, Zheng X. Effects of androgen on ultrastructure of corneal epithelium and function of the tear film in BALB/c mice. Cornea 2015;34:334–341.
[394] Appelmans M. La Kerato-conjonctivite seche de Gougerot-Sjögren. Arch Ophtalmol 1948;81:577–588.
[395] Baquiche M. Sexual dimorphism of Loewenthal's gland in albino rat. Acta Anat (Basel) 1959;36:247–280.
[396] Bizzarro A, Valentini G, Di Martino G, DaPonte A, De Bellis A, Iacono G. Influence of testosterone therapy on clinical and immunological features of autoimmune diseases associated with Klinefelter's syndrome. J Clin Endocrinol Metab 1987;64:32–36.
[397] Bruckner R. Uber einem erfolgreich mit perandren behandelten fall von Sjögren'schem symptomen komplex. Ophthalmologica 1945;110:37–42.
[398] Calmettes L, Déodati F, Planel H, Bec P. Influence des hormones génitales sur la glande lacrymale. Bull Mem Soc Fr Ophtalmol 1956;69:263–270.
[399] Carriere R. The influence of the thyroid gland on polyploid cell formation in the external orbital gland of the rat. Am J Anat 1964;115:1–15.
[400] Cavallero C. The influence of various steroids on the Lowenthal lachrymal glands of the rat. Acta Endocrinol Suppl (Copenh) 1960;51:861.
[401] Cavallero C. Relative effectiveness of varous steroids in an androgen assay using the exorbital lacrimal gland of the castrated rat. I. Delta-4-3-ketones and delta-5-3-beta-hydroxysteroids. Acta Endocrinol (Copenh) 1967;55:119–130.
[402] Cavallero C, Chiappino G, Milani F, Casella E. Uptake of 35S labelled sulfate in the exorbital lacrymal glands of adult and newborn rats under different hormonal treatment. Experientia 1960;16:429.
[403] Cavallero C, Morera P. Effect of testosterone on the nuclear volume of exorbital lacrimal glands of the white rat. Experientia 1960;16:285–286.
[404] Ducommun P, Ducommun S, Baquiche M. Comparison between the effects of 17-ethyl-19-nor-testosterone & testosterone propionate in the adult & immature rat. Acta Endocrinol (Copenh) 1959;30:78–92.
[405] Dzierzykray-Rogalska I, Chodynicki S, Wisniewski L. The effect of gonadectomy on the parotid salivary gland and Loeventhal's gland in white mice. Acta Med Pol 1963;4:221–228.
[406] Krawczuk-Hermanowiczowa O. Effect of sex hormones on the lacrimal gland. Effect of testosterone, estradiol and both hormones together on the morphological appearance of the lacrimal gland in the castrated rat. Klin Oczna 1983;85:337–339.
[407] Krawczuk-Hermanowiczowa O. Effect of sex glands on the lacrimal apparatus. II. Changes in the lacrimal apparatus of rats after castration. Klin Oczna 1983;85:15–17.
[408] Nover A. Effect of testosterone and hypophysin on tear secretion. Arzneimittelforschung 1957;7:277–278.
[409] Quintarelli G, Dellovo MC. Activation of glycoprotein biosynthesis by testesterone propionate on mouse exorbital glands. J Histochem Cytochem 1965;13:361–364.
[410] Radnot M, Nemeth B. The effect of testosterone preparations on the lacrimal glands. Orv Hetil 1954;95:580–581.
[411] Radnot M, Nemeth B. Effect of testosterone preparation on the lacrymal glands. Ophthalmologica 1955;129:376–380.
[412] Sullivan DA, Allansmith MR. Hormonal influence on the secretory immune system of the eye: endocrine interactions in the control of IgA and secretory component levels in tears of rats. Immunology 1987;60:337–343.
[413] Sullivan DA, Hann LE, Vaerman JP. Selectivity, specificity and kinetics of the androgen regulation of the ocular secretory immune system. Immunol Invest 1988;17:183–194.
[414] Ubels JL, Wertz JT, Ingersoll KE, Jackson 2nd RS, Aupperlee MD. Down-regulation of androgen receptor expression and inhibition of lacrimal gland cell proliferation by retinoic acid. Exp Eye Res 2002;75:561–571.
[415] Schonthal AH, Warren DW, Stevenson D, Schecter JE, Azzarolo AM, Mircheff AK, et al. Proliferation of lacrimal gland acinar cells in primary culture. Stimulation by extracellular matrix, EGF, and DHT. Exp Eye Res 2000;70:639–649.
[416] Gabe M. Hormonal conditioning of the morphology of the supra-parotid glands in white rats. C R Seances Soc Biol Fil 1955;149:223–225.
[417] Ebling FJ, Ebling E, Randall V, Skinner J. The effects of hypophysectomy and of bovine growth hormone on the responses to testosterone of prostate, preputial, Harderian and lachrymal glands and of brown adipose tissue in the rat. J Endocrinol 1975;66:401–406.
[418] Tsai M-J, Clark JH, Schrader WT, O'Malley BW. Mechanisms of action of hormones that act as transcription-regulatory factors. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia, PA: WB Saunders Company; 1998. p. 55–94.
[419] McPhaul MJ, Young M. Complexities of androgen action. J Am Acad Dermatol 2001;45:S87–S94.
[420] Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem 1991;266:510–518.
[421] Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, et al. The androgen receptor in health and disease. Annu Rev Physiol 2013;75:201–224.
[422] Kerkhofs S, Denayer S, Haelens A, Claessens F. Androgen receptor knockout and knock-in mouse models. J Mol Endocrinol 2009;42:11–17.
[423] Rundlett SE, Wu XP, Miesfeld RL. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol 1990;4:708–714.
[424] Mendelsohn LG. Prostate cancer and the androgen receptor: strategies for the development of novel therapeutics. Prog Drug Res 2000;55:213–233.
[425] Sullivan DA, Edwards JA, Wickham LA, Pena JD, Gao J, Ono M, et al. Identification and endocrine control of sex steroid binding sites in the lacrimal gland. Curr Eye Res 1996;15:279–291.
[426] Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand 2000;78:146–153.
[427] Rocha EM, Wickham LA, da Silveira LA, Krenzer KL, Yu FS, Toda I, et al. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol 2000;84:76–84.
[428] Aupperlee MD, Wertz JT, Ingersoll KE, Ubels JL. Identification of androgen receptors in rabbit lacrimal gland by immunohistochemsitry. Adv Exp Med Biol 2002;506:137–141.
[429] Ota M, Kyakumoto S, Nemoto T. Demonstration and characterization of cytosol androgen receptor in rat exorbital lacrimal gland. Biochem Int 1985;10:129–135.
[430] Sullivan DA. Sex hormones and Sjögren's syndrome. J Rheumatol Suppl 1997;50:17–32.
[431] Tan JA, Joseph DR, Quarmby VE, Lubahn DB, Sar M, French FS, et al. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Mol Endocrinol 1988;2:1276–1285.
[432] Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM. Autologous down-regulation of androgen receptor messenger ribonucleic acid. Mol Endocrinol 1990;4:22–28.
[433] Krongrad A, Wilson CM, Wilson JD, Allman DR, McPhaul MJ. Androgen increases androgen receptor protein while decreasing receptor mRNA in LNCaP cells. Mol Cell Endocrinol 1991;76:79–88.
[434] Mora GR, Mahesh VB. Autoregulation of the androgen receptor at the translational level: testosterone induces accumulation of androgen receptor mRNA in the rat ventral prostate polyribosomes. Steroids 1999;64:587–591.
[435] Takane KK, George FW, Wilson JD. Androgen receptor of rat penis is down-regulated by androgen. Am J Physiol 1990;258:E46–E50.
[436] Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol 1995;52:113–133.
[437] Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, et al. Testosterone signaling in T cells and macrophages. Steroids 2002;67:535–538.
[438] Zinder O, Dar DE. Neuroactive steroids: their mechanism of action and their function in the stress response. Acta Physiol Scand 1999;167:181–188.
[439] Lu SF, Mo Q, Hu S, Garippa C, Simon NG. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J Neurobiol 2003;57:163–171.
[440] Sullivan SD, Moenter SM. Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology 2003;144:4366–4375.
[441] Kelly DM, Nettleship JE, Akhtar S, Muraleedharan V, Sellers DJ, Brooke JC, et al. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci 2014;109:95–103.
[442] Zhang L, Lei D, Zhu GP, Hong L, Wu SZ. Physiological testosterone retards cardiomyocyte aging in Tfm mice via androgen receptor-independent pathway. Chin Med Sci J 2013;28:88–94.
[443] Tachibana M, Adachi W, Kinoshita S, Kobayashi Y, Honma Y, Hiai H, et al. Androgen-dependent hereditary mouse keratoconus: linkage to an MHC region. Invest Ophthalmol Vis Sci 2002;43:51–57.
[444] Karn RC, Chung AG, Laukaitis CM. Did androgen-binding protein paralogs undergo neo- and/or Subfunctionalization as the Abp gene region expanded in the mouse genome? PLoS One 2014;9:e115454.
[445] Jackson BC, Thompson DC, Wright MW, McAndrews M, Bernard A, Nebert DW, et al. Update of the human secretoglobin (SCGB) gene superfamily and an example of 'evolutionary bloom' of androgen-binding protein genes within the mouse Scgb gene superfamily. Hum Genomics 2011;5:691–702.
[446] Labrie F, Belanger A, Simard J, Van L-T, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Ann N Y Acad Sci 1995;774:16–28.
[447] Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab 1997;82:2396–2402.
[448] Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab 1994;79:1086–1090.
[449] Intracrinology Labrie F. Mol Cell Endocrinol 1991;78:C113–C118.
[450] Bélanger A. Determination of non-conjugated and conjugated steroids in human plasma. In: Gorog S, editor. 5th Symposium on the Analysis of Steroids. 1993. p. 99–110. Szombathely, Hungary.
[451] Labrie F, Belanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab 1997;82:2403–2409.
[452] Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab 1997;82:3498–3505.
[453] Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids 1998;63:322–328.
[454] Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, et al. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 1997;62:148–158.
[455] Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol 2015;145:133–138.
[456] Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res 2010;181:177–192.
[457] Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med 2004;22:299–309.
[458] Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, et al. Is dehydroepiandrosterone a hormone? J Endocrinol 2005;187:169–196.
[459] Schirra F, Suzuki T, Dickinson DP, Townsend DJ, Gipson IK, Sullivan DA. Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea 2006;25:438–442.
[460] Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988;122:552–562.
[461] Lagresa M, Suarez N, Lescaille D, Lagresa C. Inductores de lagrimas: Androgenos y gammaglobulinas humanas. Revista Cubana Oftalmol 2000;13:35–43.
[462] Worda C, Nepp J, Huber JC, Sator MO. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas 2001;37:209–212.
[463] Connor CG, Primo EJ. A weak androgenic artificial tear solution decreases the osmolarity of dry eye patients. Invest Ophthalmol Vis Sci 2001;42:S30.
[464] Song X, Zhao P, Wang G, Zhao X. The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats. Invest Ophthalmol Vis Sci 2014;55:745–751.
[465] Soria J, Duran JA, Etxebarria J, Merayo J, Gonzalez N, Reigada R, et al. Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. J Proteom 2013;78:94–112.
[466] Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar T, et al. TFOS DEWS II Tear Film Report. Ocul Surf 2017;15:366–403.
[467] Nikolov NP, Illei GG. Pathogenesis of Sjögren's syndrome. Curr Opin Rheumatol 2009;21:465–470.
[468] Valtysdottir ST, Wide L, Hallgren R. Low serum dehydroepiandrosterone sulfate in women with primary Sjögren's syndrome as an isolated sign of impaired HPA axis function. J Rheumatol 2001;28:1259–1265.
[469] Spaan M, Porola P, Laine M, Rozman B, Azuma M, Konttinen YT. Healthy human salivary glands contain a DHEA-sulphate processing intracrine machinery, which is deranged in primary Sjögren's syndrome. J Cell Mol Med 2009;13:1261–1270.
[470] Porola P, Virkki L, Przybyla BD, Laine M, Patterson TA, Pihakari A, et al. Androgen deficiency and defective intracrine processing of dehydroepiandrosterone in salivary glands in Sjögren's syndrome. J Rheumatol 2008;35:2229–2235.
[471] Laine M, Porola P, Udby L, Kjeldsen L, Cowland JB, Borregaard N, et al. Low salivary dehydroepiandrosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjögren's syndrome. Arthritis Rheum 2007;56:2575–2584.
[472] Konttinen YT, Porola P, Konttinen L, Laine M, Poduval P. Immunohistopathology of Sjögren's syndrome. Autoimmun Rev 2006;6:16–20.
[473] Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren's syndrome. Invest Ophthalmol Vis Sci 2001;42:925–932.
[474] Kolkowski E, Coll J, Roure-Mir C, Reth P, Bosch J, Pujol-Borrell, et al. Cytokine mRNA expression in minor salivary gland biopsies of primary Sjögren’s syndrome. J Rheumatol Suppl 1997;50:45.
[475] Elwaleed M, Zhu J, Deng G-M, Diab A, Link H, Klinge G, et al. Augmented levels of macrophage and Th1 cell-derived cytokine mRNA in MRL/lpr mice submandibular glands. J Rheumatol Suppl 1997;50:45.
[476] Cauli A, Yanni G, Pitzalis C, Challacombe S, Panayi GS. Cytokine and adhesion molecule expression in the minor salivary glands of patients with Sjögren's syndrome and chronic sialoadenitis. Ann Rheum Dis 1995;54:209–215.
[477] Fox P, Grisius M, Sun D, Emmert-Buck M. Cytokines in saliva and salivary glands in primary Sjögren’s syndrome. J Rheumatol Suppl 1997;50:38.
[478] Takahashi M, Ishimaru N, Yanagi K, Haneji N, Saito I, Hayashi Y. High incidence of autoimmune dacryoadenitis in male non-obese diabetic (NOD) mice depending on sex steroid. Clin Exp Immunol 1997;109:555–561.
[479] Hunger RE, Muller S, Laissue JA, Hess MW, Carnaud C, Garcia I, et al. Inhibition of submandibular and lacrimal gland infiltration in nonobese diabetic mice by transgenic expression of soluble TNF-receptor p55. J Clin Invest 1996;98:954–961.
[480] Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus 2004;13:635–638.
[481] Nestler JE. Interleukin-1 stimulates the aromatase activity of human placental cytotrophoblasts. Endocrinology 1993;132:566–570.
[482] Purohit A, Ghilchik MW, Duncan L, Wang DY, Singh A, Walker MM, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab 1995;80:3052–3058.
[483] Macdiarmid F, Wang D, Duncan LJ, Purohit A, Ghilchick MW, Reed MJ. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor alpha. Mol Cell Endocrinol 1994;106:17–21.
[484] Speirs V, Adams EF, White MC. The anti-estrogen tamoxifen blocks the stimulatory effects of interleukin-6 on 17 beta-hydroxysteroid dehydrogenase activity in MCF-7 cells. J Steroid Biochem Mol Biol 1993;46:605–611.
[485] Sokoloff MH, Tso CL, Kaboo R, Taneja S, Pang S, deKernion JB, et al. In vitro modulation of tumor progression-associated properties of hormone refractory prostate carcinoma cell lines by cytokines. Cancer 1996;77:1862–1872.
[486] Blais Y, Sugimoto K, Carriere MC, Haagensen DE, Labrie F, Simard J. Interleukin-6 inhibits the potent stimulatory action of androgens, glucocorticoids and interleukin-1 alpha on apolipoprotein D and GCDFP-15 expression in human breast cancer cells. Int J Cancer 1995;62:732–737.
[487] Ahmed SA, Talal N. Sex hormones and the immune system–Part 2. Animal data. Baillieres Clin Rheumatol 1990;4:13–31.
[488] Khalkhali-Ellis Z, Handa RJ, Price Jr. RH, Adams BD, Callaghan JJ, Hendrix MJ. Androgen receptors in human synoviocytes and androgen regulation of interleukin 1beta (IL-1beta) induced IL-6 production: a link between hypoandrogenicity and rheumatoid arthritis? J Rheumatol 2002;29:1843–1846.
[489] Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004;89:3313–3318.
[490] Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 2001;167:2060–2067.
[491] Carruba G, D'Agostino P, Miele M, Calabro M, Barbera C, Bella GD, et al. Estrogen regulates cytokine production and apoptosis in PMA-differentiated, macrophage-like U937 cells. J Cell Biochem 2003;90:187–196.
[492] Akoum A, Lemay A, Maheux R. Estradiol and interleukin-1beta exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. J Clin Endocrinol Metab 2002;87:5785–5792.
[493] Steinberg AD, Roths JB, Murphy ED, Steinberg RT, Raveche ES. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol 1980;125:871–873.
[494] Shear HL, Wofsy D, Talal N. Effects of castration and sex hormones on immune clearance and autoimmune disease in MRL/Mp-lpr/lpr and MRL/Mp-+/+ mice. Clin Immunol Immunopathol 1983;26:361–369.
[495] Brick JE, Walker SE, Wise KS. Hormone control of autoantibodies to calf thymus nuclear extract (CTE) and DNA in MRL-lpr and MRL-+/+ mice. Clin Immunol Immunopathol 1988;46:68–81.
[496] Stern M. Ocular surface inflammation: a causative factor in dry eye. J Rheumatol Suppl 1997;50:42.
[497] Gao J, Stern M. Modulators of apoptosis in the lacrimal gland of dry eye dogs. J Rheumatol Suppl 1997;50:43.
[498] Homo-Delarche F, Fitzpatrick F, Christeff N, Nunez EA, Bach JF, Dardenne M. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol 1991;40:619–637.
[499] Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev 1996;17:369–384.
[500] Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol 1995;13:217–226.
[501] Kanda N, Tsuchida T, Tamaki K. Testosterone suppresses anti-DNA antibody production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:1703–1711.
[502] Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum 1999;42:328–337.
[503] Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res 1998;47:290–301.
[504] Araneo BA, Dowell T, Diegel M, Daynes RA. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood 1991;78:688–699.
[505] Li ZG, Danis VA, Brooks PM. Effect of gonadal steroids on the production of IL-1 and IL-6 by blood mononuclear cells in vitro. Clin Exp Rheumatol 1993;11:157–162.
[506] Harbuz MS, Perveen-Gill Z, Lightman SL, Jessop DS. A protective role for testosterone in adjuvant-induced arthritis. Br J Rheumatol 1995;34:1117–1122.
[507] Cutolo M, Balleari E, Giusti M, Intra E, Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum 1991;34:1–5.
[508] Shoenfeld Y, Krause I, Blank M. New methods of treatment in an experimental murine model of systemic lupus erythematosus induced by idiotypic manipulation. Ann Rheum Dis 1997;56:5–11.
[509] Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004;117:561–574.
[510] Rahimi Darabad R, Richards SM, Sullivan DA. Sex and androgen effects on gene expression in autoimmune lacrimal glands (abstract). Invest Ophthalmol Vis Sci 2010:51. ARVO E-Abstract 4177.
[511] Richards SM, Sullivan DA. Do genetic alterations in sex steroid receptors contribute to lacrimal gland disease in Sjögren's syndrome? Open Endocrinol J 2009;3:5–11.
[512] Moutsopoulos HM. Sjögren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol 1994;72:162–165.
[513] Mostafa SE, Seamon V, Azzarolo AM. Influence of sex hormones and genetic predisposition in Sjögren’s syndrome: A new clue to the immunopathogenesis of dry eye disease. Exp Eye Res 2012;96:88–97.
[514] Mircheff AM. Understanding the causes of lacrimal insufficiency: implications for treatment and prevention of dry eye syndrome. Research to Prevent Blindness Science Writers Seminar. New York: Research to Prevent Blindness; 1993. p. 51–54.
[515] He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res 1991;19:2373–2378.
[516] Murphy L, O'Shaughnessy PJ. Testicular steroidogenesis in the testicular feminized (Tfm) mouse: loss of 17 alpha-hydroxylase activity. J Endocrinol 1991;131:443–449.
[517] Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, et al. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 2007;148:3674–3684.
[518] Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A 2006;103:224–229.
[519] Smith JA, Vitale S, Reed GF, Grieshaber SA, Goodman LA, Vanderhoof VH, et al. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol 2004;122:151–156.
[520] Greenstein BD. Lupus: why women? J Womens Health Gend Based Med 2001;10:233–239.
[521] van Blokland SC, Versnel MA. Pathogenesis of Sjögren's syndrome: characteristics of different mouse models for autoimmune exocrinopathy. Clin Immunol 2002;103:111–124.
[522] Moore PA, Bounous DI, Kaswan RL, Humphreys-Beher MG. Histologic examination of the NOD-mouse lacrimal glands, a potential model for idiopathic autoimmune dacryoadenitis in Sjögren's syndrome. Lab Anim Sci 1996;46:125–128.
[523] Humphreys-Beher MG, Hu Y, Nakagawa Y, Wang PL, Purushotham KR. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjögren's syndrome. Adv Exp Med Biol 1994;350:631–636.
[524] Hawkins T, Gala RR, Dunbar JC. The effect of neonatal sex hormone manipulation on the incidence of diabetes in nonobese diabetic mice. Proc Soc Exp Biol Med 1993;202:201–205.
[525] Rosmalen JG, Pigmans MJ, Kersseboom R, Drexhage HA, Leenen PJ, Homo-Delarche F. Sex steroids influence pancreatic islet hypertrophy and subsequent autoimmune infiltration in nonobese diabetic (NOD) and NODscid mice. Lab Invest 2001;81:231–239.
[526] Mamalis N, Harrison D, Hiura G, Hanover R, Meikle A, Warren D, et al. Dry eyes and testosterone deficiency in women (abstract). Centen Annu Meet Am Acad Ophthalmol 1996:132.
[527] Singh S, Moksha L, Sharma N, Titiyal JS, Biswas NR, Velpandian T. Development and evaluation of animal models for sex steroid deficient dry eye. J Pharmacol Toxicol Methods 2014;70:29–34.
[528] Nussbaumer P, Billich A. Steroid sulfatase inhibitors. Med Res Rev 2004;24:529–576.
[529] O'Sullivan PC, Montgomery P. Sullivan DA Ocular mucosal immunity. Handbook of Mucosal Immunology. third ed. Orlando, FL: Academic Press; 2004. p. 1477–1496.
[530] Meek B, Speijer D, de Jong PT, de Smet MD, Peek R. The ocular humoral immune response in health and disease. Prog Retin Eye Res 2003;22:391–415.
[531] Saitoh-Inagawa W, Hiroi T, Yanagita M, Iijima H, Uchio E, Ohno S, et al. Unique characteristics of lacrimal glands as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+ alphabeta T cells. Invest Ophthalmol Vis Sci 2000;41:138–144.
[532] Pabst O, Cerovic V, Hornef M. Secretory IgA in the Coordination of establishment and Maintenance of the Microbiota. Trends Immunol 2016;37:287–296.
[533] Peppard JV, Montgomery PC. Studies on the origin and composition of IgA in rat tears. Immunology 1987;62:193–198.
[534] Sullivan DA, Allansmith MR. Source of IgA in tears of rats. Immunology 1984;53:791–799.
[535] Sullivan DA. Ocular mucosal immunity. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee J, Bienenstock J, editors. Handbook of Mucosal Immunology. second ed. Orlando, FL: Academic Press; 1999. p. 1241–1281.
[536] Verrijdt G, Schoenmakers E, Alen P, Haelens A, Peeters B, Rombauts W, et al. Androgen specificity of a response unit upstream of the human secretory component gene is mediated by differential receptor binding to an essential androgen response element. Mol Endocrinol 1999;13:1558–1570.
[537] Vemuganti GK, Tiwari S, Nair RM, Vamadevan P, Ali MJ. Effect of androgens on human lacrimal gland cells in–vitro (abstract). Invest Ophthalmol Vis Sci 2016;57. ARVO E-Abstract 6194.
[538] Seal DV. The effect of ageing and disease on tear constituents. Trans Ophthalmol Soc U K 1985;104(Pt 4):355–362.
[539] Boukes RJ, Boonstra A, Breebaart AC, Reits D, Glasius E, Luyendyk L, et al. Analysis of human tear protein profiles using high performance liquid chromatography (HPLC). Doc Ophthalmol 1987;67:105–113.
[540] Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab 2000;85:4874–4882.
[541] Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab 2000;85:4866–4873.
[542] Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea 2003;22:516–521.
[543] Sullivan DA, Sullivan BD, Ullman MD, Rocha EM, Krenzer KL, Cermak JM, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci 2000;41:3732–3742.
[544] Zeligs M, Gordon K. Dehydroepiandrosterone therapy for the treatment of dry eye disorders. 1994. WO1994004155 A1. PCT/US1992/007096.
[545] Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes – a pathogenetic link between stress and acne. Exp Dermatol 2004;13(Suppl 4):31–35.
[546] Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol 2004;123:1–12.
[547] Schirra F, Suzuki T, Richards SM, Jensen RV, Liu M, Lombardi MJ, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci 2005;46:3666–3675.
[548] Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab 1993;76:524–528.
[549] Zouboulis CC, Akamatsu H, Stephanek K, Orfanos CE. Androgens affect the activity of human sebocytes in culture in a manner dependent on the localization of the sebaceous glands and their effect is antagonized by spironolactone. Skin Pharmacol 1994;7:33–40.
[550] Puy LA, Turgeon C, Gagne D, Labrie Y, Chen C, Pelletier G, et al. Localization and regulation of expression of the FAR-17A gene in the hamster flank organs. J Invest Dermatol 1996;107:44–50.
[551] Schroder HG, Ziegler M, Nickisch K, Kaufmann J, el Etreby MF. Effects of topically applied antiandrogenic compounds on sebaceous glands of hamster ears and flank organs. J Invest Dermatol 1989;92:769–773.
[552] Emanuel S. Quantitative determinations of the sebaceous glands' function, with particular mention of the method employed. Acta Derm Venereol 1936;17:444–456.
[553] Smith J, Brunot F. Hormonal effects on aged human sebaceous glands. Acta Derm Venereol 1961;41:61–65.
[554] Wirth H, Gloor M, Kimmel W. Influence of cyproterone acetate and estradiol on cell kinetics in the sebaceous gland of the golden hamster ear. Arch Dermatol Res 1980;268:277–281.
[555] Steagall RJ, Yamagami H, Wickham LA, Sullivan DA. Androgen control of gene expression in the rabbit meibomian gland. Adv Exp Med Biol 2002;506:465–476.
[556] Yamagami H, Richards SM, Sullivan BD, Liu M, Steagall RJ, Sullivan DA. Gender-associated differences in gene expression of the meibomian gland. Adv Exp Med Biol 2002;506:459–463.
[557] Yamagami H, Schirra F, Liu M, Richards SM, Sullivan BD, Sullivan DA. Androgen influence on gene expression in the meibomian gland. Adv Exp Med Biol 2002;506:477–481.
[558] Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp Eye Res 2006;83:291–296.
[559] Schirra F, Richards SM, Sullivan DA. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Curr Eye Res 2007;32:393–398.
[560] Khandelwal P, Liu S, Sullivan DA. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis 2012;18:1055–1067.
[561] Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update 2000;6:225–236.
[562] Kampa M, Pelekanou V, Castanas E. Membrane-initiated steroid action in breast and prostate cancer. Steroids 2008;73:953–960.
[563] Perra MT, Lantini MS, Serra A, Cossu M, De Martini G, Sirigu P. Human meibomian glands: a histochemical study for androgen metabolic enzymes. Invest Ophthalmol Vis Sci 1990;31:771–775.
[564] Hohl D, de Viragh PA, Amiguet-Barras F, Gibbs S, Backendorf C, Huber M. The small proline-rich proteins constitute a multigene family of differentially regulated cornified cell envelope precursor proteins. J Invest Dermatol 1995;104:902–909.
[565] http://www.ncbi.nlm.nih.gov/gene. May 24, 2016.
[566] Torres JM, Ruiz E, Ortega E. Development of a quantitative RT-PCR method to study 5alpha-reductase mRNA isozymes in rat prostate in different androgen status. Prostate 2003;56:74–79.
[567] Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol 2003;23:104–118.
[568] Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr 2001;131:2260–2268.
[569] Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999;4:611–617.
[570] Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol 2008;17:542–551.
[571] Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18:1926–1945.
[572] Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer 2006;119:757–764.
[573] Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 2006;66:7783–7792.
[574] Li DQ, Pflugfelder SC. Matrix metalloproteinases in corneal inflammation. Ocul Surf 2005;3:S198–S202.
[575] Sahin A, Kam WR, Darabad RR, Topilow K, Sullivan DA. Regulation of leukotriene B4 secretion by human corneal, conjunctival, and meibomian gland epithelial cells. Arch Ophthalmol 2012;130:1013–1018.
[576] Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, et al. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol 2000;25:1–16.
[577] Deplewski D, Rosenfield RL. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology 1999;140:4089–4094.
[578] Asano K, Maruyama S, Usui T, Fujimoto N. Regulation of estrogen receptor alpha and beta expression by testosterone in the rat prostate gland. Endocr J 2003;50:281–287.
[579] Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol 2003;17:203–208.
[580] Stoeckelhuber M, Messmer EM, Schmidt C, Xiao F, Schubert C, Klug J. Immunohistochemical analysis of secretoglobin SCGB 2A1 expression in human ocular glands and tissues. Histochem Cell Biol 2006;126:103–109.
[581] Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol 2005;20:289–298.
[582] Anderson J, Abrahamsson PA, Crawford D, Miller K, Tombal B. Management of advanced prostate cancer: can we improve on androgen deprivation therapy? BJU Int 2008;101:1497–1501.
[583] Loy CJ, Yong EL. Sex, infertility and the molecular biology of the androgen receptor. Curr Opin Obstet Gynecol 2001;13:315–321.
[584] Brinkmann AO. Molecular basis of androgen insensitivity. Mol Cell Endocrinol 2001;179:105–109.
[585] Lahita RG. The connective tissue diseases and the overall influence of gender. Int J Fertil Menopausal Stud 1996;41:156–165.
[586] Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998;17:38–56.
[587] Physicians’ Desk Reference. fiftythird Montvale, NJ: Medical Economics Co; 1999.
[588] Sullivan BD, Evans JE, Dana MR, Sullivan DA. Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol 2002;506:449–458.
[589] Bolognia JL. Aging skin. Am J Med 1995;98:99S–103S.
[590] Tamer C, Oksuz H, Sogut S. Androgen status of the nonautoimmune dry eye subtypes. Ophthalmic Res 2006;38:280–286.
[591] Suzuki T, Yokoi N, Komuro A, Kinoshita S. The cyclic change of meibomian gland physiology during the menstrual cycle (abstract). Invest Ophthalmol Vis Sci 2007;48. ARVO E-Abstract 434.
[592] Suzuki T, Yokoi N, Komuro A, Kinoshita S. Sex differences in meibomian gland physiology (abstract). ARVO 2008;49. ARVO E-Abstract 92.
[593] Schiffman RM, Bradford R, Bunnell B, Lai F, Bernstein P, Whitcup SW. A multi-center, double-masked, randomized, vehicle-controlled, parallel group study to evaluate the safety and efficacy of testosterone ophthalmic solution in patients with meibomian gland dysfunction (abstract). Invest Ophthalmol Vis Sci 2006;47. ARVO E-Abstract 5608.
[594] Hiwatari S. Protein anabolic steroids in ophthalmology. Ber Zusammenkunft Dtsch Ophthalmol Ges 1964;65:424–426.
[595] Schumacher H, Machemer R. Experimental studies on the therapy of corneal lesions due to cortisone. Klin Monbl Augenheilkd 1966;148:121–126.
[596] Hildebrandt PG. Experience in local anabolic therapy of corneal diseases. Med Monatsschr 1974;28:359–360.
[597] Yamamoto T, Terada N, Nishizawa Y, Petrow V. Angiostatic activities of medroxyprogesterone acetate and its analogues. Int J Cancer 1994;56:393–399.
[598] Schrameyer B, Busse H, Schiffer HP. Results of Nandrolone therapy (Keratyl) in lesions and diseases of the cornea (author's transl). Klin Monbl Augenheilkd 1978;173:864–871.
[599] Saruya S. Studies on allergic conjunctivitis. 5. Effects of castration and sex hormone administration on experimental allergic conjunctivitis. Nippon Ganka Gakkai Zasshi 1968;72:833–845.
[600] Ploc I, Sulcova J, Starka L. Androgen metabolism in the epithelium of the bovine cornea. Endokrinologie 1978;72:327–333.
[601] Southren AL, Altman K, Vittek J, Boniuk V, Gordon GG. Steroid metabolism in ocular tissues of the rabbit. Invest Ophthalmol 1976;15:222–228.
[602] Ploc I, Starka L. Testosterone binding in the cytosol of bovine corneal epithelium. Exp Eye Res 1979;28:111–119.
[603] Hadeyama T, Nakayasu K, Ha NT, Nakamura S. Expression of estrogen receptors alpha and beta, androgen receptors and progesterone receptors in human cornea. Nippon Ganka Gakkai Zasshi 2002;106:557–564.
[604] Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W, et al. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res 2001;22:28–33.
[605] Tachibana M, Kobayashi Y, Kasukabe T, Kawajiri K, Matsushima Y. Expression of androgen receptor in mouse eye tissues. Invest Ophthalmol Vis Sci 2000;41:64–66.
[606] Khandelwal P, Sullivan D. Androgen control of human corneal epithelial cells (abstract). . World Cornea Congress (http://slideplayercom/slide/7500598/ 2010.
[607] Mantelli F, Moretti C, Micera A, Bonini S. Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS). Graefes Arch Clin Exp Ophthalmol 2007;245:899–902.
[608] Bonini S, Mantelli F, Moretti C, Lambiase A, Bonini S, Micera A. Itchy-dry eye associated with polycystic ovary syndrome. Am J Ophthalmol 2007;143:763–771.
[609] Mantelli F, Moretti C, Macchi I, Massaro-Giordano G, Cozzupoli GM, Lambiase A, et al. Effects of sex hormones on ocular surface epithelia: Lessons learned from polycystic ovary syndrome. J Cell Physiol 2016;231:971–975.
[610] Najafi L, Malek M, Valojerdi AE, Khamseh ME, Aghaei H. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: a diagnostic accuracy study using STARD standard. J Diabetes Metab Disord 2015;14:39.
[611] Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol 2008;8:10.
[612] Seifart U, Strempel I. The dry eye and diabetes mellitus. Ophthalmologe 1994;91:235–239.
[613] Paulini K, Beneke G, Kulka R. Age- and sex-dependent changes in glandular cells. I. Histological and chemical investigations on the glandula lacrimalis, glandula orbitalis externa and glandula infraorbitalis of the rat. Gerontologia 1972;18:131–146.
[614] Tier H. Uper Zeilteilung and Kernklassen bildung in der Glandula orbitalis externa der Ratte (abstract). Acta Pathol Microbiol Scand Suppl 1944;50. Abstract #1944.
[615] Ebling FJ, Ebling E, Randall V, Skinner J. The synergistic action of alpha-melanocyte-stimulating hormone and testosterone of the sebaceous, prostate, preputial, Harderian and lachrymal glands, seminal vesicles and brown adipose tissue in the hypophysectomized-castrated rat. J Endocrinol 1975;66:407–412.
[616] Martinazzi M, Baroni C. Controllo ormonale delle ghiandola lacrimale extraorbitale nel topo con nanismo ipofisario. Folia Endocrinol 1963;16:123–132.
[617] Sullivan DA. Possible mechanisms involved in the reduced tear secretion in Sjögren’s syndrome. In: Homma M, Sugai S, Tojo T, Miyakaka N, Akizuki M, editors. Sjögren's Syndrome State of the Art. Amsterdam: Kugler Press; 1994. p. 13–19.
[618] Ng MK. New perspectives on Mars and Venus: unravelling the role of androgens in gender differences in cardiovascular biology and disease. Heart Lung Circ 2007;16:185–192.
[619] Dieudonne MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol 2006;99:189–196.
[620] Molloy EJ, O'Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 2003;102:2653–2659.
[621] MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 2008;22:2676–2689.
[622] van Nas A, Guhathakurta D, Wang SS, Yehya N, Horvath S, Zhang B, et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinolohy 2009;150:1235–1249.
[623] Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res 2005;31:91–106.
[624] Thompson AD, Angelotti T, Nag S, Mokha SS. Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience 2008;153:1268–1277.
[625] Mitsushima D, Takase K, Takahashi T, Kimura F. Activational and organisational effects of gonadal steroids on sex-specific acetylcholine release in the dorsal hippocampus. J Neuroendocrinol 2009;21:400–405.
[626] Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 1985;2006(101):1252–1261.
[627] Kipp JL, Ramirez VD. Estradiol and testosterone have opposite effects on microtubule polymerization. Neuroendocrinology 2003;77:258–272.
[628] Antus B, Yao Y, Song E, Liu S, Lutz J, Heemann U. Opposite effects of testosterone and estrogens on chronic allograft nephropathy. Transpl Int 2002;15:494–501.
[629] Cutolo M, Wilder RL. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am 2000;26:825–839.
[630] Sweezey NB, Ghibu F, Gagnon S. Sex hormones regulate CFTR in developing fetal rat lung epithelial cells. Am J Physiol 1997;272:L844–L851.
[631] Azzi L, El-Alfy M, Labrie F. Gender differences and effects of sex steroids and dehydroepiandrosterone on androgen and oestrogen alpha receptors in mouse sebaceous glands. Br J Dermatol 2006;154:21–27.
[632] Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000;21:363–392.
[633] Pochi PE, Strauss JS. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol 1974;62:191–201.
[634] Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S. Scd3–a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics 2001;71:182–191.
[635] Sullivan BD, Cermak JM, Sullivan RM, Papas AS, Evans JE, Dana MR, et al. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjögren's syndrome. Adv Exp Med Biol 2002;506:441–447.
[636] Carney F, Krumholz D, Sack R, Hall M, McCrory K, Morris CA. Key fertility hormones detected in tears correlate to systemic concentrations (abstract). Ocul Surf 2005;3:S52.
[637] Susarla R, Taylor A, Southworth HS, Sreekantam S, Williams G, Murray P, et al. Analysis of steroid metabolites using liquid chromatography with tandem mass spectrometry in biological fluids of healthy eyes (abstract). Invest Ophthalmol Vis Sci 2012;53. ARVO E-Abstract 3145.
[638] Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Davis S, et al. Local estrogen biosynthesis in males and females. Endocr Relat Cancer 1999;6:131–137.
[639] Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 2003;86:225–230.
[640] Spelsberg H, Klueppel M, Reinhard T, Glaeser M, Niederacher D, Beckmann MW, et al. Detection of oestrogen receptors (ER) alpha and beta in conjunctiva, lacrimal gland, and tarsal plates. Eye (Lond) 2004;18:729–733.
[641] Fuchsjager-Mayrl G, Nepp J, Schneeberger C, Sator M, Dietrich W, Wedrich A, et al. Identification of estrogen and progesterone receptor mRNA expression in the conjunctiva of premenopausal women. Invest Ophthalmol Vis Sci 2002;43:2841–2844.
[642] Esmaeli B, Harvey JT, Hewlett B. Immunohistochemical evidence for estrogen receptors in meibomian glands. Ophthalmology 2000;107:180–184.
[643] Auw-Haedrich C, Feltgen N. Estrogen receptor expression in meibomian glands and its correlation with age and dry-eye parameters. Graefes Arch Clin Exp Ophthalmol 2003;241:705–709.
[644] Gligorijevic J, Krstic M, Babic G. Immunohistochemical detection of estrogen and progesterone receptors in the human lacrimal gland. Arch Biol Sci 2011;63:319–324.
[645] Kam W, Darabad R, Sullivan D. Membrane steroid receptors are expressed by human meibomian gland epithelial cells (abstract). Invest Ophthalmol Vis Sci 2014;55. ARVO E-Abstract 17.
[646] Suzuki T, Schirra F, Richards S, Jensen R, Sullivan D. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci 2008;49:1797–1808.
[647] Sullivan DA. Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf 2004;2:92–123.
[648] Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science 2005;307:1572–1573.
[649] Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 2003;4:46–56.
[650] Ho KJ, Liao JK. Nonnuclear actions of estrogen. Arterioscler Thromb Vasc Biol 2002;22:1952–1961.
[651] Lange CA. Editorial: membrane and nuclear steroid hormone receptors: two integrated arms of the same signaling pathway? Steroids 2007;72:105–106.
[652] Wendler A, Albrecht C, Wehling M. Nongenomic actions of aldosterone and progesterone revisited. Steroids 2012;77:1002–1006.
[653] Wehling M, Schultz A, Losel R. Nongenomic actions of estrogens: exciting opportunities for pharmacology. Maturitas 2006;54:321–326.
[654] Kam W, Sullivan D. Suppressive effects of 17β-estradiol on immortalized human meibomian gland epithelial cells (abstract). Invest Ophthalmol Vis Sci 2013;54. E-Abstract 4316.
[655] Lang TJ. Estrogen as an immunomodulator. Clin Immunol 2004;113:224–230.
[656] Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574.
[657] Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015;294:63–69.
[658] Trigunaite A, Dimo J, Jorgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol 2015;294:87–94.
[659] Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One 2012;7:e44552.
[660] Chen RY, Fan YM, Zhang Q, Liu S, Li Q, Ke GL, et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORgammaT transcription by recruiting the ERalpha/REA complex to estrogen response elements of the RORgammaT promoter. J Immunol 2015;194:4019–4028.
[661] Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II Pathophysiology report. Ocul Surf 2017;15:438–510.
[662] Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol 2011;187:2386–2393.
[663] Sullivan D, Jensen R, Suzuki T, Richards S. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis 2009;15:1553–1572.
[664] Schechter JE, Warren DW, Mircheff AK. A lacrimal gland is a lacrimal gland, but rodent's and rabbit's are not human. Ocul Surf 2010;8:111–134.
[665] Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013;110:3507–3512.
[666] Azzarolo A, Eihausen H, Schechter J. Estrogen prevention of lacrimal gland cell death and lymphocytic infiltration. Exp Eye Res 2003;77:347–354.
[667] Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren's syndrome through fas-mediated apoptosis. Am J Pathol 1999;155:173–181.
[668] Turaka K, Nottage JM, Hammersmith KM, Nagra PK, Rapuano CJ. Dry eye syndrome in aromatase inhibitor users. Clin Exp Ophthalmol 2013;41:239–243.
[669] Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 2014;383:1041–1048.
[670] Inglis H, Boyle FM, Friedlander ML, Watson SL. Dry eyes and AIs: If you don't ask you won't find out. Breast 2015;24:694–698.
[671] Zylberberg C, Seamon V, Ponomareva O, Vellala K, Deighan M, Azzarolo AM. Estrogen up-regulation of metalloproteinase-2 and -9 expression in rabbit lacrimal glands. Exp Eye Res 2007;84:960–972.
[672] Sullivan D, Allansmith M. Hormonal influence on the secretory immune system of the eye: androgen modulation of IgA levels in tears of rats. J Immunol 1985;134:2978–2982.
[673] Sullivan D, Bloch K, Allansmith M. Hormonal influence on the secretory immune system of the eye: androgen regulation of secretory component levels in rat tears. J Immunol 1984;132:1130–1135.
[674] Uncu G, Avci R, Uncu Y, Kaymaz C, Develioglu O. The effects of different hormone replacement therapy regimens on tear function, intraocular pressure and lens opacity. Gynecol Endocrinol 2006;22:501–505.
[675] Sator MO, Joura EA, Golaszewski T, Gruber D, Frigo P, Metka M, et al. Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br J Obstet Gynaecol 1998;105:100–102.
[676] Akramian J, Wedrich A, Nepp J, Sator M. Estrogen therapy in keratoconjunctivitis sicca. Adv Exp Med Biol 1998;438:1005–1009.
[677] Altintas O, Caglar Y, Yuksel N, Demirci A, Karabas L. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica 2004;218:120–129.
[678] Guaschino S, Grimaldi E, Sartore A, Mugittu R, Mangino F, Bortoli P, et al. Visual function in menopause: the role of hormone replacement therapy. Menopause 2003;10:53–57.
[679] Affinito P, Di Spiezio Sardo A, Di Carlo C, Sammartino A, Tommaselli GA, Bifulco G, et al. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause 2003;10:482–487.
[680] Moon J, Jung J, Shin K, Paik H. Effect of hormone replacement therapy on dry eye syndrome in postmenopausal women: A prospective study. J Korean Ophthalmol Soc 2010;51:175–179.
[681] Coksuer H, Ozcura F, Oghan F, Haliloglu B, Coksuer C. Effects of estradiol-drospirenone on ocular and nasal functions in postmenopausal women. Climacteric 2011;14:482–487.
[682] Okon A, Jurowski P, Gos R. [The influence of the hormonal replacement therapy on conjunctival epithelium morphology among peri- and postmenopausal women]. Klin Oczna 2001;103:183–186.
[683] Feldman F, Bain J, Matuk AR. Daily assessment of ocular and hormonal variables throughout the menstrual cycle. Arch Ophthalmol 1978;96:1835–1838.
[684] Sansone-Bazzano G, Reisner RM, Bazzano G. A possible mechanism of action of estrogen at the cellular level in a model sebaceous gland. J Invest Dermatol 1972;59:299–304.
[685] Ebling FJ, Skinner J. The local effects of topically applied estradiol, cyproterone acetate, and ethanol on sebaceous secretion in intact male rats. J Invest Dermatol 1983;81:448–451.
[686] Guy R, Ridden C, Kealey T. The improved organ maintenance of the human sebaceous gland: modeling in vitro the effects of epidermal growth factor, androgens, estrogens, 13-cis retinoic acid, and phenol red. J Invest Dermatol 1996;106:454–460.
[687] Pochi PE. Acne: endocrinologic aspects. Cutis 1982;30:212–214. 6-7, 9 passim.
[688] Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev 2012;7:CD004425.
[689] Golebiowski B, Badarudin N, Eden J, You J, Hampel U, Stapleton F. Does endogenous serum oestrogen play a role in Meibomian gland dysfunction in postmenopausal women with dry eye. Br J Ophthalmol 2016;101:218–222.
[690] Suzuki T, Sullivan DA. Comparative effects of estrogen on matrix metalloproteinases and cytokines in immortalized and primary human corneal epithelial cell cultures. Cornea 2006;25:454–459.
[691] Suzuki T, Sullivan DA. Estrogen stimulation of proinflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea 2005;24:1004–1009.
[692] Wang C, Shi X, Chen X, Wu H, Zhang H, Xie J, et al. 17-beta-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol Vis 2012;18:1115–1122.
[693] Oh TH, Chang DJ, Choi JS, Joo CK. Effects of 17beta-estradiol on human corneal wound healing in vitro. Cornea 2012;31:1158–1164.
[694] Millodot M. Effect of soft lenses on corneal senstitvity. Acta Ophthalmol 1974;52:603–608.
[695] Vavilis D, Maloutas S, Nasioutziki M, Boni E, Bontis J. Conjunctiva is an estrogen-sensitive epithelium. Acta Obstet Gynecol Scand 1995;74:799–802.
[696] Vavilis D, Agorastos T, Vakiani M, Jafetas J, Panidis D, Konstantinidis T, et al. The effect of transdermal estradiol on the conjunctiva in postmenopausal women. Eur J Obstet Gynecol Reprod Biol 1997;72:93–96.
[697] Pelit A, Bagis T, Kayaselcuk F, Dursun D, Akova Y, Aydin P. Tear function tests and conjunctival impression cytology before and after hormone replacement therapy in postmenopausal women. Eur J Ophthalmol 2003;13:337–342.
[698] Tomlinson A, Pearce EI, Simmons PA, Blades K. Effect of oral contraceptives on tear physiology. Ophthalmic Physiol Opt 2001;21:9–16.
[699] Chen SP, Massaro-Giordano G, Pistilli M, Schreiber CA, Bunya VY. Tear osmolarity and dry eye symptoms in women using oral contraception and contact lenses. Cornea 2013;32:423–428.
[700] Boga A, Golebiowski B, Stapleton F. Development of the 2-item Daily Ocular Symptoms Survey to assess day-to-day fluctuations in discomfort and dryness (abstract). Invest Ophthalmol Vis Sci 2016. E-abstract 2857.
[701] Tatlipinar S, Gedik S, Irkec M, Orhan M, Erdener U. Ocular ferning during the menstrual cycle in healthy women. Eur J Ophthalmol 2001;11:15–18.
[702] Erdem U, Muftuoglu O, Goktolga U, Dagli S. Effect of hormone replacement therapy in women on ocular refractive status and aberrations. J Refract Surg 2007;23:567–572.
[703] Shaharuddin B, Ismail-Mokhtar S, Hussein E. Dry eye in post-menopausal Asian women on hormone replacement therapy. Int J Ophthalmol 2008;1:158–160.
[704] Evans V, Millar TJ, Eden JA, Willcox MD. Menopause, hormone replacement therapy and tear function. Adv Exp Med Biol 2002;506:1029–1033.
[705] Jensen AA, Higginbotham EJ, Guzinski GM, Davis IL, Ellish NJ. A survey of ocular complaints in postmenopausal women. J Assoc Acad Minor Phys 2000;11:44–49.
[706] Okon A, Jurowski P, Gos R. [The influence of the hormonal replacement therapy on the amount and stability of the tear film among peri- and postmenopausal women]. Klin Oczna 2001;103:177–181.
[707] Kuscu NK, Toprak AB, Vatansever S, Koyuncu FM, Guler C. Tear function changes of postmenopausal women in response to hormone replacement therapy. Maturitas 2003;44:63–68.
[708] Piwkumsribonruang N, Somboonporn W, Luanratanakorn P, Kaewrudee S, Tharnprisan P, Soontrapa S. Effectiveness of hormone therapy for treating dry eye syndrome in postmenopausal women: a randomized trial. J Med Assoc Thai 2010;93:647–652.
[709] Akramian J, Ries E, Krepler K, Nepp J, Sator M, Wedrich A. Estrogen therapy in keratoconjunctivitis sicca. Spektrum Augenheilkd 1997;11:195–197.
[710] Kemdinum Idu F, Osita Emina M, Oyem Ubaru C. Tear secretion and tear stability of women on hormonal contraceptives. J Optom 2013;6:45–50.
[711] Nagler RM, Pollack S. Sjögren's syndrome induced by estrogen therapy. Semin Arthritis Rheum 2000;30:209–214.
[712] Erdem U, Ozdegirmenci O, Sobaci E, Sobaci G, Goktolga U, Dagli S. Dry eye in post-menopausal women using hormone replacement therapy. Maturitas 2007;56:257–262.
[713] Taner P, Akarsu C, Atasoy P, Bayram M, Ergin A. The effects of hormone replacement therapy on ocular surface and tear function tests in postmenopausal women. Ophthalmologica 2004;218:257–259.
[714] Lekskul A, Nimvorapun T, Manonai J, Hanutsaha P, Tasanee Sirikul S. The study of tear function, central corneal thickness and corneal endothelial cell count in postmenopausal hormone replacement therapy. Thai J Ophthalmol 2004;18:121–127.
[715] Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 2004;16:857–872.
[716] Rauz S, Walker EA, Murray PI, Stewart PM. Expression and distribution of the serum and glucocorticoid regulated kinase and the epithelial sodium channel subunits in the human cornea. Exp Eye Res 2003;77:101–108.
[717] Margolis RN, Evans RM, O'Malley BW, ; Consortium NA.. The nuclear receptor signaling atlas: Development of a functional atlas of nuclear receptors. Mol Endocrinol 2005;19:2433–2436.
[718] Stallcup MR, Kim JH, Teyssier C, Lee YH, Ma H, Chen D. The roles of protein-protein interactions and protein methylation in transcriptional activation by nuclear receptors and their coactivators. J Steroid Biochem Mol Biol 2003;85:139–145.
[719] Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 2003;17:1681–1692.
[720] Rogatsky I, Zarember KA, Yamamoto KR. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 2001;20:6071–6083.
[721] Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci U S A 2002;99:16701–16706.
[722] Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 2005;353:1711–1723.
[723] Aragona P, Aguennouz M, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 2015;122:62–71.
[724] Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology 1999;106:811–816.
[725] Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol 2003;136:593–602.
[726] Aragona P, Spinella R, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren syndrome. Eur J Ophthalmol 2013;23:368–376.
[727] Susarla R, Liu L, Walker EA, Bujalska IJ, Alsalem J, Williams GP, et al. Cortisol biosynthesis in the human ocular surface innate immune response. PLoS One 2014;9:e94913.
[728] Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, et al. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet 2003;34:434–439.
[729] Stewart PM. 11 beta-Hydroxysteroid dehydrogenase: implications for clinical medicine. Clin Endocrinol (Oxf) 1996;44:493–499.
[730] White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 1997;18:135–156.
[731] Freeman L, Hewison M, Hughes SV, Evans KN, Hardie D, Means TK, et al. Expression of 11beta-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 2005;106:2042–2049.
[732] Gilmour JS, Coutinho AE, Cailhier JF, Man TY, Clay M, Thomas G, et al. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J Immunol 2006;176:7605–7611.
[733] Wilson SE, Lloyd SA, Kennedy RH. Fibroblast growth factor receptor-1, interleukin-1 receptor, and glucocorticoid receptor messenger RNA production in the human lacrimal gland. Invest Ophthalmol Vis Sci 1993;34:1977–1982.
[734] Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal 2010;3:ra74.
[735] Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci 2014;1317:1–6.
[736] Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol 1995;27:9–20.
[737] Jahn R, Padel U, Porsch PH, Soling HD. Adrenocorticotropic hormone and alpha-melanocyte-stimulating hormone induce secretion and protein phosphorylation in the rat lacrimal gland by activation of a cAMP-dependent pathway. Eur J Biochem 1982;126:623–629.
[738] Leiba H, Garty NB, Schmidt-Sole J, Piterman O, Azrad A, Salomon Y. The melanocortin receptor in the rat lacrimal gland: a model system for the study of MSH (melanocyte stimulating hormone) as a potential neurotransmitter. Eur J Pharmacol 1990;181:71–82.
[739] Cripps MM, Bromberg BB, Patchen-Moor K, Welch MH. Adrenocorticotropic hormone stimulation of lacrimal peroxidase secretion. Exp Eye Res 1987;45:673–682.
[740] Entwistle ML, Hann LE, Sullivan DA, Tatro JB. Characterization of functional melanotropin receptors in lacrimal glands of the rat. Peptides 1990;11:477–483.
[741] Sullivan DA. Influence of the hypothalamic-pituitary axis on the androgen regulation of the ocular secretory immune system. J Steroid Biochem 1988;30:429–433.
[742] Martinazzi M. Effects of hypophysectomy on the extra orbital lacrimal gland of the rat. Folia Endocrinol Mens Incretologia Incretoterapia 1962;15:120–129.
[743] Minam A, Kamel T. Sur la glande lacrymale extérieure chez le rat et ses modifications aprés hypophysectomie. Compt Rend Séanc Soc Biol 1959;153:269–273.
[744] Wegelius O, Friman C. Two different pituitary-controlled sulphation mechanisms in the rat. Acta Med Scand 1964;175:221–228.
[745] Srikantan S, Paliwal A, Quintanar-Stephano A, De PK. Estrogen and androgen repression of two female specific lacrimal lipocalins in hamster: Pituitary independent and sex hormone receptor mediated action. Gen Comp Endocrinol 2007;151:172–179.
[746] Pochin EE. The mechanism of experimental exophthalmos caused by pituitary extracts. Ciba Found Coll Endocrinol 1952;4:316–326.
[747] Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci 2013;54:2541–2550.
[748] McClellan KA, Robertson FG, Kindblom J, Wennbo H, Tornell J, Bouchard B, et al. Investigation of the role of prolactin in the development and function of the lacrimal and harderian glands using genetically modified mice. Invest Ophthalmol Vis Sci 2001;42:23–30.
[749] Casbon AJ, Warren DW, Mircheff AK. Prolactin inhibits carbachol-dependent secretion by lacrimal acinar cells in vitro. Adv Exp Med Biol 2002;506:231–235.
[750] Schechter J, Carey J, Wallace M, Wood R. Distribution of growth factors and immune cells are altered in the lacrimal gland during pregnancy and lactation. Exp Eye Res 2000;71:129–142.
[751] Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA Ophthalmol 2014;132:593–599.
[752] Mircheff AK, Warren DW, Wood RL, Tortoriello PJ, Kaswan RL. Prolactin localization, binding, and effects on peroxidase release in rat exorbital lacrimal gland. Invest Ophthalmol Vis Sci 1992;33:641–650.
[753] Selvam S, Mircheff AK, Yiu SC. Diverse mediators modulate the chloride ion fluxes that drive lacrimal fluid production. Invest Ophthalmol Vis Sci 2013;54:2927–2933.
[754] Araujo AS, Simoes Mde J, Verna C, Simoes RS, Soares Junior JM, Baracat EC, et al. Influence of hyperprolactinemia on collagen fibers in the lacrimal gland of female mice. Clinics (Sao Paulo) 2015;70:632–637.
[755] Frey WH, Nelson JD, Frick ML, Elde RP. Prolactin immunoreactivity in human tears and lacrimal gland: Possible implications for tear production. In: Holly FJ, editor. The preocular tear film in health, disease and contact lens wear. Lubbock, TX: Dry Eye Institute; 1986. p. 798–807.
[756] Wood RL, Park KH, Gierow JP, Mircheff AK. Immunogold localization of prolactin in acinar cells of lacrimal gland. Adv Exp Med Biol 1994;350:75–77.
[757] Markoff E, Lee D, Fellows J. Human lacrimal glands synthesize and release prolactin (abstract). Endocrinology 1995;152:440A.
[758] Wood RL, Zhang J, Huang ZM, Gierow JP, Schechter JE, Mircheff AK, et al. Prolactin and prolactin receptors in the lacrimal gland. Exp Eye Res 1999;69:213–226.
[759] Zhang J, Whang G, Gierow J. Prolactin receptors are present in rabbit lacrimal gland (abstract). Invest Ophthalmol Vis Sci 1995;36(Suppl):S991. S991. 1995;36.
[760] Wang Y, Chiu CT, Nakamura T, Walker AM, Petridou B, Trousdale MD, et al. Traffic of endogenous, transduced, and endocytosed prolactin in rabbit lacrimal acinar cells. Exp Eye Res 2007;85:749–761.
[761] Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthalmol Vis Sci 2012;53:6738–6747.
[762] Warren DW, Platler BW, Azzarolo AM, Huang ZM, Wang G, Wood RL, et al. Pilocarpine stimulates prolactin secretion by rabbit lacrimal glands (abstract). Invest Ophthalmol Vis Sci 1995;36:S651.
[763] Azzarolo AM, Kaswan RL, Huang ZM, Platler BW, Mircheff AK, Warren DW. Is lacrimal gland prolactin content regulated? Abstr Invest Ophthalmol Vis Sci 1995;36:S651.
[764] Narukawa S, Kanzaki H, Inoue T, Imai K, Higuchi T, Hatayama H, et al. Androgens induce prolactin production by human endometrial stromal cells in vitro. J Clin Endocrinol Metab 1994;78:165–168.
[765] Nevalainen MT, Martikainen P, Valve EM, Ping W, Nurmi M, Harkonen PL. Prolactin regulation of rat and human prostate (abstract). In: 12th International Symposium of the Journal of Steroid Biochemistry & Molecular Biology. 1995. p. 53.
[766] Wilder RL. Adrenal and gonadal steroid hormone deficiency in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl 1996;44:10–12.
[767] Ormandy CJ, Clarke CL, Kelly PA, Sutherland RL. Androgen regulation of prolactin-receptor gene expression in MCF-7 and MDA-MB-453 human breast cancer cells. Int J Cancer 1992;50:777–782.
[768] Mathers WD, Stovall D, Lane JA, Zimmerman MB, Johnson S. Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea 1998;17:353–358.
[769] Reber PM. Prolactin and immunomodulation. Am J Med 1993;95:637–644.
[770] McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol 1991;147:3780–3787.
[771] Elbourne KB, Keisler D, McMurray RW. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus 1998;7:420–427.
[772] Sahin OG, Kartal E, Taheri N. Meibomian gland dysfunction: endocrine aspects. ISRN Ophthalmol 2011;2011:465198.
[773] Duenas Z, Torner L, Corbacho AM, Ochoa A, Gutierrez-Ospina G, Lopez-Barrera F, et al. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest Ophthalmol Vis Sci 1999;40:2498–2505.
[774] Hann LE, Tatro JB, Sullivan DA. Morphology and function of lacrimal gland acinar cells in primary culture. Invest Ophthalmol Vis Sci 1989;30:145–158.
[775] Tatro JB, Entwistle ML. Heterogeneity of brain melanocortin receptors suggested by differential ligand binding in situ. Brain Res 1994;635:148–158.
[776] Akbulut S, Byersdorfer CA, Larsen CP, Zimmer SL, Humphreys TD, Clarke BL. Expression of the melanocortin 5 receptor on rat lymphocytes. Biochem Biophys Res Commun 2001;281:1086–1092.
[777] Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997;91:789–798.
[778] Salomon Y, Zohar M, Dejordy JO, Eshel Y, Shafir I, Leiba H, et al. Signaling mechanisms controlled by melanocortins in melanoma, lacrimal, and brain astroglial cells. Ann N Y Acad Sci 1993;680:364–380.
[779] Gilbard JP, Rossi SR, Heyda KG, Dartt DA. Stimulation of tear secretion by topical agents that increase cyclic nucleotide levels. Invest Ophthalmol Vis Sci 1990;31:1381–1388.
[780] Trousdale MD, Stevenson D, Zhu Z, Kaslow HR, Schechter JE, Warren DW, et al. Effect of anti-inflammatory cytokines on the activation of lymphocytes by lacrimal gland acinar cells in an autologous mixed cell reaction. Adv Exp Med Biol 2002;506:789–794.
[781] Ru Y, Huang Y, Liu H, Du J, Meng Z, Dou Z, et al. alpha-Melanocyte-stimulating hormone ameliorates ocular surface dysfunctions and lesions in a scopolamine-induced dry eye model via PKA-CREB and MEK-Erk pathways. Sci Rep 2015;5:18619.
[782] Atmaca M, Kizildag E, Candan Z, Ozbay MF, Seven I. Ocular findings in Sheehan's syndrome. Graefes Arch Clin Exp Ophthalmol 2015;253:759–763.
[783] Khong PL, Peh WC, Low LC, Leong LL. Variant of the Triple A syndrome. Australas Radiol 1994;38:222–224.
[784] Heinrichs C, Tsigos C, Deschepper J, Drews R, Collu R, Dugardeyn C, et al. Familial adrenocorticotropin unresponsiveness associated with alacrima and achalasia: biochemical and molecular studies in two siblings with clinical heterogeneity. Eur J Pediatr 1995;154:191–196.
[785] Tsigos C, Arai K, Latronico AC, DiGeorge AM, Rapaport R, Chrousos GP. A novel mutation of the adrenocorticotropin receptor (ACTH-R) gene in a family with the syndrome of isolated glucocorticoid deficiency, but no ACTH-R abnormalities in two families with the triple A syndrome. J Clin Endocrinol Metab 1995;80:2186–2189.
[786] Chavez M, Moreno C, Perez A, Garcia F, Solis J, Cargone A, et al. Allgrove syndrome (achalasia-alacrima-adrenal gland insufficiency): report of a case. Rev Gastroenterol Peru 1996;16:153–157.
[787] Varkonyi A, Julesz J, Szuts P, Toth I, Faredin I. Simultaneous occurrence of selective ACTH deficiency, achalasia, alacrimia and hyperlipoproteinemia. Orv Hetil 1990;131:2763–2766.
[788] Dugardeyn C, Anooshiravani M, Christophe C, Goyens P, Perlmutter N. Achalasia-alacrima-ACTH insensitivity syndrome (Triple-A-syndrome). J Belge Radiol 1993;76:167–168.
[789] Huebner A, Yoon SJ, Ozkinay F, Hilscher C, Lee H, Clark AJ, et al. Triple A syndrome–clinical aspects and molecular genetics. Endocr Res 2000;26:751–759.
[790] Ziangirova GG, Bocharov VE, Malaeva LV, Olinevich VB, Sherstnev VV. Effect of ACTH4-10 on the course of inflammatory and regeneration processes in the rabbit cornea. Vestn Oftalmol 1997;113:26–29.
[791] Scheving LE, Tsai TH, Pauly JE, Halberg F. Circadian effect of ACTH 1-17 on mitotic index of the corneal epithelium of BALB/C mice. Peptides 1983;4:183–190.
[792] Horwath-Winter J, Flogel I, Ramschak-Schwarzer S, Hofer A, Kroisel PM. Psoriasis and hypogonadism in chronic blepharokeratoconjunctivitis. A case report. Ophthalmologe 2002;99:380–383.
[793] Eckstein AK, Finkenrath A, Heiligenhaus A, Renzing-Kohler K, Esser J, Kruger C, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand 2004;82:291–297.
[794] Djeridane Y. Immunohistochemical evidence for the presence of vasopressin in the rat harderian gland, retina and lacrimal gland. Exp Eye Res 1994;59:117–120.
[795] Bar RS, Harrison LC, Muggeo M, Gorden P, Kahn CR, Roth J. Regulation of insulin receptors in normal and abnormal physiology in humans. Adv Intern Med 1979;24:23–52.
[796] Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995;16:3–34.
[797] Miquet JG, Gonzalez L, Matos MN, Hansen CE, Louis A, Bartke A, et al. Transgenic mice overexpressing GH exhibit hepatic upregulation of GH-signaling mediators involved in cell proliferation. J Endocrinol 2008;198:317–330.
[798] Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology 2011;152:2546–2551.
[799] Frank SJ, Gilliland G, Kraft AS, Arnold CS. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 1994;135:2228–2239.
[800] Wang X, Darus CJ, Xu BC, Kopchick JJ. Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol Endocrinol 1996;10:1249–1260.
[801] Kopchick JJ, Andry JM. Growth hormone (GH), GH receptor, and signal transduction. Mol Genet Metab 2000;71:293–314.
[802] Humbel RE. Insulin-like growth factors I and II. Eur J Biochem 1990;190:445–462.
[803] Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004;14:395–403.
[804] Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 1996;15:6541–6551.
[805] Bevan P. Insulin signalling. J Cell Sci 2001;114:1429–1430.
[806] Varewijck AJ, Janssen JA. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer 2012;19:F63–F75.
[807] Steele-Perkins G, Turner J, Edman JC, Hari J, Pierce SB, Stover C, et al. Expression and characterization of a functional human insulin-like growth factor I receptor. J Biol Chem 1988;263:11486–11492.
[808] Frattali AL, Pessin JE. Relationship between alpha subunit ligand occupancy and beta subunit autophosphorylation in insulin/insulin-like growth factor-1 hybrid receptors. J Biol Chem 1993;268:7393–7400.
[809] Germain-Lee EL, Janicot M, Lammers R, Ullrich A, Casella SJ. Expression of a type I insulin-like growth factor receptor with low affinity for insulin-like growth factor II. Biochem J 1992;281:413–417.
[810] Narawane MA, Lee VH. IGF-I and EGF receptors in the pigmented rabbit bulbar conjunctiva. Curr Eye Res 1995;14:905–910.
[811] Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJA, Velloso LA. Identification of insulin in the tear film and insulin receptor and IGF-I receptor on the human ocular surface. Invest Ophthalmol Vis Sci 2002;43:963–967.
[812] Rocha EM, de MLMH, Carvalho CR, Saad MJ, Velloso LA. Characterization of the insulin-signaling pathway in lacrimal and salivary glands of rats. Curr Eye Res 2000;21:833–842.
[813] Burren CP, Berka JL, Edmondson SR, Werther GA, Batch JA. Localization of mRNAs for insulin-like growth factor-I (IGF-I), IGF-I receptor, and IGF binding proteins in rat eye. Invest Ophthalmol Vis Sci 1996;37:1459–1468.
[814] Arnold DR, Moshayedi P, Schoen TJ, Jones BE, Chader GJ, Waldbillig RJ. Distribution of IGF-I and -II, IGF binding proteins (IGFBPs) and IGFBP mRNA in ocular fluids and tissues: potential sites of synthesis of IGFBPs in aqueous and vitreous. Exp Eye Res 1993;56:555–565.
[815] Ding J, Wirostko B, Sullivan DA. Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea 2015;34:686–692.
[816] Ciresi A, Amato MC, Morreale D, Lodato G, Galluzzo A, Giordano C. Cornea in acromegalic patients as a possible target of growth hormone action. J Endocrinol Invest 2011;34. e30–5.
[817] Parentin F, Pensiero S. Central corneal thickness in children with growth hormone deficiency. Acta Ophthalmol 2010;88:692–694.
[818] Quaranta L, Riva I, Mazziotti G, Porcelli T, Floriani I, Katsanos A, et al. Elevated intraocular pressure in patients with acromegaly. Graefes Arch Clin Exp Ophthalmol 2014;252:1133–1139.
[819] Ciresi A, Morreale R, Radellini S, Cillino S, Giordano C. Corneal thickness in children with growth hormone deficiency: the effect of GH treatment. Growth Horm IGF Res 2014;24:150–154.
[820] Ozkok A, Hatipoglu E, Tamcelik N, Balta B, Gundogdu AS, Ozdamar MA, et al. Corneal biomechanical properties of patients with acromegaly. Br J Ophthalmol 2014;98:651–657.
[821] Altinkaynak H, Duru N, Ersoy R, Kalkan Akcay E, Ugurlu N, Cagil N, et al. Topographic and biomechanical evaluation of cornea in patients with acromegaly. Cornea 2015;34:65–70.
[822] Sen E, Tutuncu Y, Balikoglu-Yilmaz M, Elgin U, Berker D, Ozturk F, et al. Corneal biomechanical properties measured by the ocular response analyzer in acromegalic patients. Graefes Arch Clin Exp Ophthalmol 2014;252:1283–1288.
[823] Kebapcilar AG, Tatar MG, Ipekci SH, Gonulalan G, Korkmaz H, Baldane S, et al. Cornea in PCOS patients as a possible target of IGF-1 action and insulin resistance. Arch Gynecol Obstet 2014;290:1255–1263.
[824] Wu YC, Buckner BR, Zhu M, Cavanagh HD, Robertson DM. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul Surf 2012;10:100–107.
[825] Liu Y, Ding J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci 2014;55:5596–5601.
[826] Ding J, Liu Y, Sullivan DA. Effects of insulin and high glucose on human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci 2015;56:7814–7820.
[827] Shamsheer RP, Arunachalam C. A clinical study of meibomian gland dysfunction in patients with diabetes. Middle East Afr J Ophthalmol 2015;22:462–466.
[828] Cunha DA, Carneiro EM, Alves Mde C, Jorge AG, de Sousa SM, Boschero AC, et al. Insulin secretion by rat lacrimal glands: effects of systemic and local variables. Am J Physiol Endocrinol Metab 2005;289:E768–E775.
[829] Cunha DA, de Alves MC, Stoppiglia LF, Jorge AG, Modulo CM, Carneiro EM, et al. Extra-pancreatic insulin production in rat lachrymal gland after streptozotocin-induced islet beta-cells destruction. Biochim Biophys Acta 2007;1770:1128–1135.
[830] Malki LT, Dias AC, Jorge AG, Modulo CM, Rocha EM. Lacrimal gland primary acinar cell culture: the role of insulin. Arq Bras Oftalmol 2016;79:105–110.
[831] Shetty R, Saeed T, Rashed H, Adeghate E, Singh J. Effect of diabetes mellitus on acinar morphology, peroxidase concentration, and release in isolated rat lacrimal glands. Curr Eye Res 2009;34:905–911.
[832] Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci 2012;53:8067–8074.
[833] Dias AC, Batista TM, Roma LP, Modulo CM, Malki LT, Dias LC, et al. Insulin replacement restores the vesicular secretory apparatus in the diabetic rat lacrimal gland. Arq Bras Oftalmol 2015;78:158–163.
[834] Adeghate E, Ponery A, Hameed R. Notes on the effect of diabetes mellitus on the morphology and function of the rat lacrimal gland (abstract). Ocul Surf 2005;3:S42.
[835] Alves M, Calegari VC, Cunha DA, Saad MJ, Velloso LA, Rocha EM. Increased expression of advanced glycation end-products and their receptor, and activation of nuclear factor kappa-B in lacrimal glands of diabetic rats. Diabetologia 2005;48:2675–2681.
[836] Modulo CM, Jorge AG, Dias AC, Braz AM, Bertazolli-Filho R, Jordao Jr. AA, et al. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocrine 2009;36:161–168.
[837] Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 2006;20:2613–2629.
[838] Ling G, Sugathan A, Mazor T, Fraenkel E, Waxman DJ. Unbiased, genome-wide in vivo mapping of transcriptional regulatory elements reveals sex differences in chromatin structure associated with sex-specific liver gene expression. Mol Cell Biol 2010;30:5531–5544.
[839] Meinhardt UJ, Ho KK. Modulation of growth hormone action by sex steroids. Clin Endocrinol (Oxf) 2006;65:413–422.
[840] Jorgensen JO, Christensen JJ, Krag M, Fisker S, Ovesen P, Christiansen JS. Serum insulin-like growth factor I levels in growth hormone-deficient adults: influence of sex steroids. Horm Res 2004;62(Suppl 1):73–76.
[841] Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol 2004;151(Suppl 3):U95–U100.
[842] Godsland IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, et al. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism 1993;42:846–853.
[843] Bertoli A, De Pirro R, Fusco A, Greco AV, Magnatta R, Lauro R. Differences in insulin receptors between men and menstruating women and influence of sex hormones on insulin binding during the menstrual cycle. J Clin Endocrinol Metab 1980;50:246–250.
[844] Coksuer H, Ozcura F, Oghan F, Haliloglu B, Karatas S. Effects of hyperandrogenism on tear function and tear drainage in patients with polycystic ovary syndrome. J Reprod Med 2011;56:65–70.
[845] Gonzalez-Ortiz M, Martinez-Abundis E, Lifshitz A. Insulin sensitivity and sex steroid hormone levels during the menstrual cycle in healthy women with non-insulin-dependent diabetic parents. Gynecol Obstet Invest 1998;46:187–190.
[846] De Paiva CS, Rocha EM. Sjögren syndrome: what and where are we looking for? Curr Opin Ophthalmol 2015;26:517–525.
[847] Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 1980;29:1–13.
[848] Makino S, Kunimoto K, Muraoka Y, Katagiri K. Effect of castration on the appearance of diabetes in NOD mouse. Jikken Dobutsu 1981;30:137–140.
[849] Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 1996;15:1292–1300.
[850] Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem 1998;273:31308–31316.
[851] Arici C, Hatipoglu E, Iskeleli G, Sultan P, Yuksel C, Gundogdu S, et al. Tear osmolarity and tear function changes in patients with acromegaly. Curr Eye Res 2015;40:863–869.
[852] Mavragani CP, Fragoulis GE, Moutsopoulos HM. Endocrine alterations in primary Sjögren's syndrome: an overview. J Autoimmun 2012;39:354–358.
[853] Katz J, Stavropoulos F, Cohen D, Robledo J, Stewart C, Heft M. IGF-1 and insulin receptor expression in the minor salivary gland tissues of Sjögren's syndrome and mucoceles–immunohistochemical study. Oral Dis 2003;9:7–13.
[854] Markopoulos AK, Poulopoulos AK, Kayavis I, Papanayotou P. Immunohistochemical detection of insulin-like growth factor-I in the labial salivary glands of patients with Sjögren's syndrome. Oral Dis 2000;6:31–34.
[855] de Wilde MA, Goverde AJ, Veltman-Verhulst SM, Eijkemans MJ, Franx A, Fauser BC, et al. Insulin action in women with polycystic ovary syndrome and its relation to gestational diabetes. Hum Reprod 2015;30:1447–1453.
[856] Bosco C, Crawley D, Adolfsson J, Rudman S, Van Hemelrijck M. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PLoS One 2015;10:e0117344.
[857] Dati E, Baroncelli GI, Mora S, Russo G, Baldinotti F, Parrini D, et al. Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex Dev 2009;3:188–193.
[858] Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23:713–719.
[859] Aydin Y, Hassa H, Burkankulu D, Arslantas D, Sayiner D, Ozerdogan N. What is the risk of metabolic syndrome in adolescents with normal BMI who have polycystic ovary syndrome? J Pediatr Adolesc Gynecol 2015;28:271–274.
[860] Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 2005;11:2063–2073.
[861] Rocha EM, Fernandes MLA, Velloso LA. Insulin signaling in the aging nervous system. Adv Cell Aging Gerontol 2004;16:107–132.
[862] Alves M, Cunha DA, Calegari VC, Saad MJ, Boschero AC, Velloso LA, et al. Nuclear factor-kappaB and advanced glycation end-products expression in lacrimal glands of aging rats. J Endocrinol 2005;187:159–166.
[863] Rocha EM, Carvalho CR, Saad MJ, Velloso LA. The influence of ageing on the insulin signalling system in rat lacrimal and salivary glands. Acta Ophthalmol Scand 2003;81:639–645.
[864] Batista TM, Tomiyoshi LM, Dias AC, Roma LP, Modulo CM, Malki LT, et al. Age-dependent changes in rat lacrimal gland anti-oxidant and vesicular related protein expression profiles. Mol Vis 2012;18:194–202.
[865] Tsubota K, Kawashima M, Inaba T, Dogru M, Matsumoto Y, Ishida R, et al. The antiaging approach for the treatment of dry eye. Cornea 2012;31(Suppl 1):S3–S8.
[866] Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003;299:572–574.
[867] Smith Jr. DL, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing research. Eur J Clin Invest 2010;40:440–450.
[868] Chandrasekar B, McGuff HS, Aufdermorte TB, Troyer DA, Talal N, Fernandes G. Effects of calorie restriction on transforming growth factor beta 1 and proinflammatory cytokines in murine Sjögren's syndrome. Clin Immunol Immunopathol 1995;76:291–296.
[869] Wang C, Peng Y, Pan S, Li L. Effect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett 2014;558:169–174.
[870] Akinci A, Cetinkaya E, Aycan Z. Dry eye syndrome in diabetic children. Eur J Ophthalmol 2007;17:873–878.
[871] Imam S, Elagin RB, Jaume JC. Diabetes-associated dry eye syndrome in a new humanized transgenic model of type 1 diabetes. Mol Vis 2013;19:1259–1267.
[872] Grus FH, Sabuncuo P, Dick HB, Augustin AJ, Pfeiffer N. Changes in the tear proteins of diabetic patients. BMC Ophthalmol 2002;2:4.
[873] Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001;108:586–592.
[874] Dogru M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol 2000;84:1210.
[875] Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol 2000;84:19–21.
[876] Alves M, Reinach PS, Paula JS, Vellasco e Cruz AA, Bachette L, Faustino J, et al. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PLoS One 2014;9:e97921.
[877] Walsh NP, Fortes MB, Raymond-Barker P, Bishop C, Owen J, Tye E, et al. Is whole-body hydration an important consideration in dry eye? Invest Ophthalmol Vis Sci 2012;53:6622–6627.
[878] Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg 1994;220:19–24.
[879] Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Ann Surg 1995;221:56–59. 649–56; discussion.
[880] Decker D, Springer W, Tolba R, Lauschke H, Hirner A, von Ruecker A. Perioperative treatment with human growth hormone down-regulates apoptosis and increases superoxide production in PMN from patients undergoing infrarenal abdominal aortic aneurysm repair. Growth Horm IGF Res 2005;15:193–199.
[881] Garcia-Esteo F, Pascual G, Garcia-Honduvilla N, Gallardo A, San Roman J, Bellon JM, et al. Histological evaluation of scar tissue inflammatory response: the role of hGH in diabetic rats. Histol Histopathol 2005;20:53–57.
[882] Garcia-Esteo F, Pascual G, Gallardo A, San-Roman J, Bujan J, Bellon JM. A biodegradable copolymer for the slow release of growth hormone expedites scarring in diabetic rats. J Biomed Mater Res B Appl Biomater 2007;81:291–304.
[883] Wirostko B, Rafii M, Sullivan DA, Morelli J, Ding J. Novel therapy to treat corneal epithelial defects: A hypothesis with growth hormone. Ocul Surf 2015;13:204–212. e1.
[884] Berthaut A, Mirshahi P, Benabbou N, Ducros E, Agra A, Therwath A, et al. Insulin growth factor promotes human corneal fibroblast network formation in vitro. Invest Ophthalmol Vis Sci 2011;52:7647–7653.
[885] Etheredge L, Kane BP, Valkov N, Adams S, Birk DE, Hassell JR. Enhanced cell accumulation and collagen processing by keratocytes cultured under agarose and in media containing IGF-I, TGF-beta or PDGF. Matrix Biol 2010;29:519–524.
[886] Izumi K, Kurosaka D, Iwata T, Oguchi Y, Tanaka Y, Mashima Y, et al. Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing. Invest Ophthalmol Vis Sci 2006;47:591–598.
[887] Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res 2003;77:581–592.
[888] Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res 1998;17:79–87.
[889] Trosan P, Svobodova E, Chudickova M, Krulova M, Zajicova A, Holan V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev 2012;21:3341–3350.
[890] Ueno H, Hattori T, Kumagai Y, Suzuki N, Ueno S, Takagi H. Alterations in the corneal nerve and stem/progenitor cells in diabetes: preventive effects of insulin-like growth factor-1 treatment. Int J Endocrinol 2014;2014:312401.
[891] Feldman ST, Gately D, Seely BL, Schonthal A, Feramisco JR. Stimulation of DNA synthesis and c-fos expression in corneal endothelium by insulin or insulin-like growth factor-I. Invest Ophthalmol Vis Sci 1993;34:2105–2111.
[892] Hyldahl L. Control of cell proliferation in the human embryonic cornea: an autoradiographic analysis of the effect of growth factors on DNA synthesis in endothelial and stromal cells in organ culture and after explantation in vitro. J Cell Sci 1986;83:1–21.
[893] Choi SH, Kay EP, Oh DS, Gu X, Smith RE. Insulin-like growth factor-I promotes cell proliferation in the absence of modulation of collagen phenotypes and utilizes IRS-1, not PLC-gamma 1, in corneal endothelial cells. Curr Eye Res 1995;14:669–676.
[894] Yamada N, Yanai R, Inui M, Nishida T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Invest Ophthalmol Vis Sci 2005;46:833–839.
[895] Yamada N, Yanai R, Kawamoto K, Nagano T, Nakamura M, Inui M, et al. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Invest Ophthalmol Vis Sci 2006;47:3286–3292.
[896] Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol 1996;169:159–166.
[897] Nagano T, Nakamura M, Nakata K, Yamaguchi T, Takase K, Okahara A, et al. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci 2003;44:3810–3815.
[898] Nakamura M, Kawahara M, Nakata K, Nishida T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Invest Ophthalmol Vis Sci 2003;44:2937–2940.
[899] Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia 2003;46:839–842.
[900] Nakamura M, Ofuji K, Chikama T, Nishida T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res 1997;16:275–278.
[901] Chikamoto N, Chikama T, Yamada N, Nishida T, Ishimitsu T, Kamiya A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn J Ophthalmol 2009;53:464–469.
[902] Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol 2008;92:896–900.
[903] Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol 2007;51:442–447.
[904] Yanai R, Nishida T, Chikama T, Morishige N, Yamada N, Sonoda KH. Potential New Modes of Treatment of Neurotrophic Keratopathy. Cornea 2015;34(Suppl 11):S121–S127.
[905] Nakamura M, Chikama T, Nishida T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr Eye Res 2005;30:825–834.
[906] Robertson DM, Zhu M, Wu YC. Cellular distribution of the IGF-1R in corneal epithelial cells. Exp Eye Res 2012;94:179–186.
[907] Wu YC, Zhu M, Robertson DM. Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells. PLoS One 2012;7:e42483.
[908] Lee HK, Lee JH, Kim M, Kariya Y, Miyazaki K, Kim EK. Insulin-like growth factor-1 induces migration and expression of laminin-5 in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci 2006;47:873–882.
[909] Ofuji K, Nakamura M, Nishida T. Signaling regulation for synergistic effects of substance P and insulin-like growth factor-1 or epidermal growth factor on corneal epithelial migration. Jpn J Ophthalmol 2000;44:1–8.
[910] McDermott AM, Kern TS, Reid TW, Russell P, Murphy CJ. Effect of substance P, insulin-like growth factor-1 and vasoactive intestinal polypeptide on corneal re-epithelialization in galactosemic rats. Curr Eye Res 1998;17:1143–1149.
[911] Hassell JR, Kane BP, Etheredge LT, Valkov N, Birk DE. Increased stromal extracellular matrix synthesis and assembly by insulin activated bovine keratocytes cultured under agarose. Exp Eye Res 2008;87:604–611.
[912] Ko JA, Yanai R, Nishida T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J Cell Physiol 2009;221:254–261.
[913] Soltau JB, McLaughlin BJ. Effects of growth factors on wound healing in serum-deprived kitten corneal endothelial cell cultures. Cornea 1993;12:208–215.
[914] Lyu J, Lee KS, Joo CK. Transactivation of EGFR mediates insulin-stimulated ERK1/2 activation and enhanced cell migration in human corneal epithelial cells. Mol Vis 2006;12:1403–1410.
[915] Yanai R, Yamada N, Inui M, Nishida T. Correlation of proliferative and anti-apoptotic effects of HGF, insulin, IGF-1, IGF-2, and EGF in SV40-transformed human corneal epithelial cells. Exp Eye Res 2006;83:76–83.
[916] Shanley LJ, McCaig CD, Forrester JV, Zhao M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci 2004;45:1088–1094.
[917] Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, et al. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci 2005;46:3604–3615.
[918] Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type I diabetic rats. Exp Eye Res 2009;89:686–692.
[919] Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol 2007;125:1082–1088.
[920] Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes 2006;55:1141–1147.
[921] Naeser P. Insulin receptors in human ocular tissues. Immunohistochemical demonstration in normal and diabetic eyes. Ups J Med Sci 1997;102:35–40.
[922] Ishiko S, Yoshida A, Mori F, Abiko T, Kitaya N, Konno S, et al. Corneal and lens autofluorescence in young insulin-dependent diabetic patients. Ophthalmologica 1998;212:301–305.
[923] Bueno EM, Saeidi N, Melotti S, Ruberti JW. Effect of serum and insulin modulation on the organization and morphology of matrix synthesized by bovine corneal stromal cells. Tissue Eng Part A 2009;15:3559–3573.
[924] Musselmann K, Kane B, Alexandrou B, Hassell JR. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci 2006;47:5260–5266.
[925] Bartlett JD, Slusser TG, Turner-Henson A, Singh KP, Atchison JA, Pillion DJ. Toxicity of insulin administered chronically to human eye in vivo. J Ocul Pharmacol 1994;10:101–107.
[926] Bartlett JD, Turner-Henson A, Atchison JA, Woolley TW, Pillion DJ. Insulin administration to the eyes of normoglycemic human volunteers. J Ocul Pharmacol 1994;10:683–690.
[927] Hatou S, Yamada M, Akune Y, Mochizuki H, Shiraishi A, Joko T, et al. Role of insulin in regulation of Na+-/K+-dependent ATPase activity and pump function in corneal endothelial cells. Invest Ophthalmol Vis Sci 2010;51:3935–3942.
[928] Schultz G, Cipolla L, Whitehouse A, Eiferman R, Woost P, Jumblatt M. Growth factors and corneal endothelial cells: III. Stimulation of adult human corneal endothelial cell mitosis in vitro by defined mitogenic agents. Cornea 1992;11:20–27.
[929] Crow JM, Lima PH, Agapitos PJ, Nelson JD. Effects of insulin and EGF on DNA synthesis in bovine endothelial cultures: flow cytometric analysis. Invest Ophthalmol Vis Sci 1994;35:128–133.
[930] Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst 2013;18:306–315.
[931] Saragas S, Arffa R, Rabin B, Kronish J, Miller D, Mayman C. Reversal of wound strength retardation by addition of insulin to corticosteroid therapy. Ann Ophthalmol 1985;17:428–430.
[932] Woost PG, Brightwell J, Eiferman RA, Schultz GS. Effect of growth factors with dexamethasone on healing of rabbit corneal stromal incisions. Exp Eye Res 1985;40:47–60.
[933] Leite SC, de Castro RS, Alves M, Cunha DA, Correa ME, da Silveira LA, et al. Risk factors and characteristics of ocular complications, and efficacy of autologous serum tears after haematopoietic progenitor cell transplantation. Bone Marrow Transpl 2006;38:223–227.
[934] Mac Cord Medina F, Silvestre de Castro R, Leite SC, Rocha EM, de Melo Rocha G. Management of dry eye related to systemic diseases in childhood and longterm follow-up. Acta Ophthalmol Scand 2007;85:739–744.
[935] Rocha EM, Pelegrino FSA, de Paiva CS, Vigorito AC, de Souza CA. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transpl 2000;25:1101–1103.
[936] Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy report. Ocul Surf 2017;15:575–628.
[937] Shantha TR, Shantha EM, Shantha JG. New methods of treating dry eye syndrome. Google Patent No.: US 2012/0003296A1; 2012.
[938] Rocha EM, Boschero AC, Cunha DA, Carneiro EM, Velloso LA, Saad MJ. Eyedrop formula of insulin for dry eye treatment. 2004. PI0401186–4.
[939] Lopez RF, Rocha EM, Cruz EL, Cazarim MS. Microparticles, methods of production, ophthalmic composition, and use. 2015. BR 1020150058560. Brazil.
[940] Storckenfeldt L, Schofield PN, Engstrom W. Stimulatory effect of insulin like growth factor II on DNA synthesis in the human embryonic cornea. Cell Biol Int Rep 1991;15:1217–1223.
[941] Musselmann K, Kane BP, Alexandrou B, Hassell JR. IGF-II is present in bovine corneal stroma and activates keratocytes to proliferate in vitro. Exp Eye Res 2008;86:506–511.
[942] Bohnsack RN, Warejcka DJ, Wang L, Gillespie SR, Bernstein AM, Twining SS, et al. Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing. Invest Ophthalmol Vis Sci 2014;55:7697–7708.
[943] Jiang Y, Ju Z, Zhang J, Liu X, Tian J, Mu G. Effects of insulin-like growth factor 2 and its receptor expressions on corneal repair. Int J Clin Exp Pathol 2015;8:10185–10191.
[944] Dias AC, Modulo CM, Jorge AG, Braz AM, Jordao Jr. AA, Filho RB, et al. Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest Ophthalmol Vis Sci 2007;48:3038–3042.
[945] Thomas O, Mahe M, Campion L, Bourdin S, Milpied N, Brunet G, et al. Long-term complications of total body irradiation in adults. Int J Radiat Oncol Biol Phys 2001;49:125–131.
[946] Brent GA. The molecular basis of thyroid hormone action. N Engl J Med 1994;331:847–853.
[947] Sawas-Dimopoulou C, Papanastasiou E, Angelis A, Toubanakis N, Margaritis L. Tissue localization of [125I]triiodothyronine in the periorbital area of mice: a microautoradiographic study. Int J Rad Appl Instrum B 1992;19:627–637.
[948] Coll J, Anglada J, Tomas S, Reth P, Goday A, Millan M, et al. High prevalence of subclinical Sjögren's syndrome features in patients with autoimmune thyroid disease. J Rheumatol 1997;24:1719–1724.
[949] Wang TJ, Wang IJ, Hu CC, Lin HC. Comorbidities of dry eye disease: a nationwide population-based study. Acta Ophthalmol 2012;90:663–668.
[950] Rocha EM, Mantelli F, Nominato LF, Bonini S. Hormones and dry eye syndrome: an update on what we do and don't know. Curr Opin Ophthalmol 2013;24:348–355.
[951] D'Arbonneau F, Ansart S, Le Berre R, Dueymes M, Youinou P, Pennec YL. Thyroid dysfunction in primary Sjögren's syndrome: a long-term followup study. Arthritis Rheum 2003;49:804–809.
[952] Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 1996;121:284–290.
[953] Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology 1996;103:958–962.
[954] Achtsidis V, Tentolouris N, Theodoropoulou S, Panagiotidis D, Vaikoussis E, Saldana M, et al. Dry eye in Graves ophthalmopathy: correlation with corneal hypoesthesia. Eur J Ophthalmol 2013;23:473–479.
[955] Hoffman RA, Wertz P, Habeeb P. Harderian glands of golden hamsters: morphological and biochemical responses to thyroid hormones. J Comp Physiol B 1989;159:293–299.
[956] Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 1993;14:184–193.
[957] Goolamali SK, Evered D, Shuster S. Thyroid disease and sebaceous function. Br Med J 1976;1:432–433.
[958] McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol (Oxf) 2008;69:687–696.
[959] Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol (Copenh) 1983;61:108–116.
[960] Matheis N, Grus FH, Breitenfeld M, Knych I, Funke S, Pitz S, et al. Proteomics differentiate between thyroid-associated orbitopathy and dry eye syndrome. Invest Ophthalmol Vis Sci 2015;56:2649–2656.
[961] Short SE, Yang YC, Jenkins TM. Sex, gender, genetics, and health. Am J Public Health 2013;103(Suppl 1):S93–S101.
[962] Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health 2013;13:677.
[963] Wallace PM, Suzuki R. Regional, racial, and gender differences in colorectal cancer screening in middle-aged African-Americans and Whites. J Cancer Educ 2012;27:703–708.
[964] Vasquez C, Lioznov D, Nikolaenko S, Yatsishin S, Lesnikova D, Cox D, et al. Gender disparities in HIV risk behavior and access to health care in St. Petersburg, Russia. AIDS Patient Care STDS 2013;27:304–310.
[965] Parent MC, Torrey C, Michaels MS. HIV testing is so gay”: the role of masculine gender role conformity in HIV testing among men who have sex with men. J Couns Psychol 2012;59:465–470.
[966] Yue L, Yu PM, Zhao DH, Wu DY, Zhu GX, Wu XY, et al. Determinants of quality of life in people with epilepsy and their gender differences. Epilepsy Behav 2011;22:692–696.
[967] Elliott JO, Mares AS. Gender differences in quality of life among Canadian adults with epilepsy. Epilepsy Res 2012;100:42–48.
[968] Colella TJ, Gravely S, Marzolini S, Grace SL, Francis JA, Oh P, et al. Sex bias in referral of women to outpatient cardiac rehabilitation? A meta-analysis. Eur J Prev Cardiol 2015;22:423–441.
[969] Thomas JF, Temple JR, Perez N, Rupp R. Ethnic and gender disparities in needed adolescent mental health care. J Health Care Poor Underserved 2011;22:101–110.
[970] Gibbons SW, Barnett SD, Hickling EJ, Herbig-Wall PL, Watts DD. Stress, coping, and mental health-seeking behaviors: gender differences in OEF/OIF health care providers. J Trauma Stress 2012;25:115–119.
[971] Chapa DW, Akintade B, Thomas SA, Friedmann E. Gender differences in stroke, mortality, and hospitalization among patients with atrial fibrillation: A systematic review. Heart Lung 2015;44:189–198.
[972] Shrestha AD, Kosalram K, Gopichandran V. Gender difference in care of type 2 diabetes. JNMA J Nepal Med Assoc 2013;52:245–250.
[973] Peek ME. Gender differences in diabetes-related lower extremity amputations. Clin Orthop Relat Res 2011;469:1951–1955.
[974] Schiebinger L, Stefanick ML. Gender matters in biological research and medical practice. J Am Coll Cardiol 2016;67:136–138.
[975] Courtright P, West SK. Contribution of sex-linked biology and gender roles to disparities with trachoma. Emerg Infect Dis 2004;10:2012–2016.
[976] Pili K, Kastelan S, Karabatic M, Kasun B, Culig B. Dry eye in contact lens wearers as a growing public health problem. Psychiatr Danub 2014;26(Suppl 3):528–532.
[977] Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci 2006;47:1319–1328.
[978] Cumberland PM, Chianca A, Rahi JS, Eyes UKB, Vision C. Laser refractive surgery in the UK Biobank study: Frequency, distribution by sociodemographic factors, and general health, happiness, and social participation outcomes. J Cataract Refract Surg 2015;41:2466–2475.
[979] Wang B, Naidu RK, Chu R, Dai J, Qu X, Zhou H. Dry eye disease following refractive surgery: A 12-month follow-up of SMILE versus FS-LASIK in high myopia. J Ophthalmol 2015;2015:132417.
[980] Shtein RM. Post-LASIK dry eye. Expert Rev Ophthalmol 2011;6:575–582.
[981] Levitt AE, Galor A, Weiss JS, Felix ER, Martin ER, Patin DJ, et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disorders. Mol Pain 2015;11:21.
[982] Dohlman TH, Lai EC, Ciralsky JB. Dry eye disease after refractive surgery. Int Ophthalmol Clin 2016;56:101–110.
[983] Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II Iatrogenic report. Ocul Surf 2017;15:511–538.
[984] Wyatt KD, Branda ME, Inselman JW, Ting HH, Hess EP, Montori VM, et al. Genders of patients and clinicians and their effect on shared decision making: a participant-level meta-analysis. BMC Med Inf Decis Mak 2014;14:81.
[985] Deepmala D, Franz L, Aponte C, Agrawal M, Jiang W. Identification of provider characteristics influencing prescription of analgesics: a systematic literature review. Pain Pract 2013;13:504–513.
[986] Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012;13:859–866.
[987] Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain 2012;13:228–234.
[988] Vierhaus M, Lohaus A, Schmitz AK. Sex, gender, coping, and self-efficacy: mediation of sex differences in pain perception in children and adolescents. Eur J Pain 2011;15(621):e1–8.
[989] Schmitz AK, Vierhaus M, Lohaus A. Pain tolerance in children and adolescents: sex differences and psychosocial influences on pain threshold and endurance. Eur J Pain 2013;17:124–131.
[990] Institute of Medicine (US) Committee on Advancing Pain Research Care and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press (US); 2011.
[991] Racine M, Castarlenas E, de la Vega R, Tome-Pires C, Sole E, Miro J, et al. Sex differences in psychological response to pain in patients with fibromyalgia syndrome. Clin J Pain 2015;31:425–432.
[992] Otto MW, Dougher MJ. Sex differences and personality factors in responsivity to pain. Percept Mot Skills 1985;61:383–390.
[993] Liu NJ, Schnell S, Wessendorf MW, Gintzler AR. Sex, pain, and opioids: interdependent influences of sex and pain modality on dynorphin-mediated antinociception in rats. J Pharmacol Exp Ther 2013;344:522–530.
[994] Hagiwara H, Ishida M, Arita J, Mitsushima D, Takahashi T, Kimura F, et al. The cAMP response element-binding protein in the bed nucleus of the stria terminalis modulates the formalin-induced pain behavior in the female rat. Eur J Neurosci 2009;30:2379–2386.
[995] Hagiwara H, Funabashi T, Akema T, Kimura F. Sex-specific differences in pain response by dopamine in the bed nucleus of the stria terminalis in rats. Neuroreport 2013;24:181–185.
[996] Acosta-Rua AJ, Cannon RL, Yezierski RP, Vierck CJ. Sex differences in effects of excitotoxic spinal injury on below-level pain sensitivity. Brain Res 2011;1419:85–96.
[997] Yu HY, Tang FI, Yeh MC, Kuo BI, Yu S. Use, perceived effectiveness, and gender differences of pain relief strategies among the community-dwelling elderly in Taiwan. Pain Manag Nurs 2011;12:41–49.
[998] Ono R, Higashi T, Takahashi O, Tokuda Y, Shimbo T, Endo H, et al. Sex differences in the change in health-related quality of life associated with low back pain. Qual Life Res 2012;21:1705–1711.
[999] Bernardes SF, Costa M, Carvalho H. Engendering pain management practices: the role of physician sex on chronic low-back pain assessment and treatment prescriptions. J Pain 2013;14:931–940.
[1000] Veldhuijzen DS, Karhof S, Leenders ME, Karsch AM, van Wijck AJ. Impact of physicians' sex on treatment choices for low back pain. Pain Pract 2013;13:451–458.
Table 1 Summary of barriers to progress in research on sex differences. back to top
|
Item
|
Barrier
|
|
Terminology use in literature
|
- •Inconsistent use of the terms sex and gender
|
|
Research tools and resources
|
- •Studies with more complex experimental designs, model systems and additional resources are lacking.
- •Information on sex differences and sex of origin of cell and tissue culture material is lacking in the literature.
- •Lack of data from longitudinal studies about different diseases, disorders, and conditions across the life span.
- •Lack of consideration of endocrine status or hormonal variability.
|
|
Interdisciplinary and collaborative research
|
- •Interpretation and application of federal regulations is not uniform.
- •Interdisciplinary research, training and translational research is lacking in the study of sex differences.
|
|
Non-health-related implications of research on sex differences in health
|
- •Lack of awareness that the consequences of genetics and physiology may be open to change.
- •Discrimination based on identified sex differences.
|
Data are adapted from the Institute of Medicine report [3].
Table 2 Sex differences in the prevalence of DED in epidemiological studies in Asian populations. back to top
|
Country
|
N
|
Definition
|
% higher risk in females
|
|
China [41]
|
1816
|
≥1 of 6 symptoms at least “often”
|
0%
|
|
China [42]
|
2262
|
≥3 of 7 symptoms at least “sometimes”
|
200%
|
|
China [43]
|
2009
|
≥1 of 6 symptoms at least “often”
|
56%
|
|
China [44]
|
1840
|
≥1 of 6 symptoms at least “often”
|
0%
|
|
Indonesia [45]
|
1058
|
≥1 of 6 symptoms at least “often’
|
43% greater in males than females
|
|
Japan [46]
|
598
|
Self-report based on symptoms
|
21%
|
|
Japan [47]
|
3433
|
Both dryness and irritation “constantly” or “often”
|
16%
|
|
Japan [48]
|
3549
|
Both dryness and irritation “constantly” or “often”
|
78%
|
|
Japan [49]
|
2644
|
Both dryness and irritation “constantly” or “often”
|
63%
|
|
Japan [50]
|
561
|
Japanese criteria
|
>200%
|
|
Singapore [51]
|
1004
|
≥1 of 5 symptoms at least “often”
|
64%
|
|
Singapore [52]
|
3280
|
≥1 of 6 symptoms at least “often”
|
67% higher in males
|
|
South Korea [53]
|
11,666
|
Dryness or irritation
|
93%
|
|
South Korea [54]
|
657
|
≥1 of 6 symptoms at least “often”
|
36%
|
|
South Korea [55]
|
16,431
|
Self-reported DED diagnosis
|
280%
|
|
Taiwan [56]
|
1361
|
≥1 of 6 symptoms at least “often”
|
30%
|
Table 3 Sex-related differences in the anatomy, physiology and pathophysiology of the lacrimal gland, cornea, conjunctiva, meibomian gland, nasolacrimal duct and tear film. back to top
|
Male
|
Female
|
Species
|
|
Lacrimal gland
|
|
|
- More frequent palpebral lobe periductal fibrosis & focal atrophy in elderly
- Higher median area of acini
- Higher absolute weight
|
- Decrease in area & thickness during aging
- More frequent orbital lobe diffuse fibrosis, periductal fibrosis & diffuse atrophy in elderly
- Higher incidence of focal adenitis (especially in females > 45 years old)
- Higher prevalence of bilateral disease
- Higher volume by MRI measurement
|
Human [14,192–198]
|
- Large and irregular acini with wide lumina
- Cell borders either indistinct or not apparent
- Glandular epithelial cells with cloudy, light granular & basophilic cytoplasm
- Centrally located nucleus varying substantially in size & shape
- Marked nuclear polymorphism
- Increased number of polyploid nuclei
- Nuclei frequently harbor prominent nucleoli
- Basal vacuoles and higher number of intranuclear inclusions in acinar cells
- Sparse intercellular channels
- Specialized structure of Golgi fields
- Capillary endothelia display few pores
- Enhanced labeling index of epithelial cells suggesting decreased cell turnover during aging
|
- Smaller, more regular acini with narrow lumina
- Acinar cell contours more conspicuous
- Cell borders clear & lobulated
- Epithelial cells with clearer and somewhat structureless cytoplasm with heavy basophilic staining around nucleus (lighter toward periphery)
- Basally-located nucleus showing more regularity in size & shape
- Many large cytoplasmic vesicles
- Frequent intercellular channels
- Capillary endothelia typically show pores
- Higher incidence of glandular autoimmune disease
- Higher acinar density
- More severe fibrosis during aging
- Increased number of intercalated, intralobular and interlobular ducts, especially in older animals
- Higher collagen content in periacinar and periductal regions of older animals
- Higher susceptibility to cytomegalovirus invasion and/or replication
- Higher mast cell numbers
- Greater expression of more than 1000 mRNAs (e.g. for androgen receptor, bcl-2, c-myc, c-myb, p53, IL-1? and TNF-?, asialoglycoprotein receptor & pancreatic lipase-related protein 1)
- Multiple sex-specific responses in gene expression to hormone exposure
- Higher synthesis of various proteins (e.g. melatonin, 20 kDa protein & N-acetyltransferase, exocrine-gland-secreting peptide 36, lacrimal gland peroxidase, as well as leucine aminopeptidase after puberty)
- Higher secretion of various proteins (e.g. 20 kDa & 90 kDa proteins)
- Greater amounts of melatonin & N-acetyltransferase
- Higher specific activity of Na+, K + -ATPase, cholinergic receptors, acid and alkaline phosphatase & galactosyltransferase
|
Mouse, rat, guinea pig, rabbit [11,34,64–109,199–205]
|
- Increased acinar area during aging
- More striking sexual dimorphism during aging
- Greater extent of acinar metaplasia & harderianization
- Higher activity of hydroxyindole-o-methyltransferase & carbonic anhydrase
- Higher phenylephrine-induced secretion of peroxidase and total protein by gland in vitro
- Higher insulin-induced insulin receptor phosphorylation
- Increased number of IgA-containing cells after puberty
- Higher number of lymphocyte foci
- Greater expression of more than 1000 mRNAs (e.g. for ?2 micro-globulin, secretory component [SC], cystatin-related protein, TGF-?1, Fas antigen, cysteine-rich secretory proteins-1 & -3, mouse urinary protein, lipophilin AL2 & androgen-binding protein subunits)
- Multiple sex-specific responses in gene expression to hormone exposure
- Higher production of various proteins (e.g. androgen receptor, immunoglobulin A [IgA] & SC, lipocalin, exocrine-gland-secreting peptide 1, submandibular androgen-repressed protein)
- Higher secretion of various proteins (e.g. SC, IgA, cystatin-related protein, 42 kDa & 46 kDa proteins)
- Higher number & affinity of ?-adrenergic binding sites
|
|
- Greater total quantity of ?-adrenergic receptors
- Increased acinar cell lipid accumulation, and higher carbachol and pilocarpine-induced lacrimal gland secretion in the absence of P2X7 receptor
|
|
|
|
|
|
Meibomian gland
|
|
|
- Higher levels of 137 mRNAs (e.g. lysozyme, prolactin-induced protein)
- Higher expression of specific polar lipids in meibum in both young and old men
- Higher expression of specific neutral lipids in meibum in old men
- Higher expression of specific fatty acid products, predominantly polar, in meibum than age-matched women
- Higher casual level of meibum in young men
- Higher incidence of abnormal lid margin and gland dropout in men ?70 years-old
- Greater decrease in symptoms following LipiFlow thermal pulsation treatment
- Higher prevalence of asymptomatic MGD
|
- Higher levels of 120 mRNAs (e.g. S100 calcium-binding protein, leptin)
- Higher expression of specific polar lipids in meibum in both young and old women
- Higher expression of specific neutral lipids in meibum in old women
- Higher expression of specific fatty acid products, predominantly polar, in meibum
- Higher prevalence of meibomitis-related keratoconjunctivitis
|
Human [7,10,110–116]
|
|
|
- Multiple sex-specific responses in gene expression to hormone exposure
- Greater levels of more than 1000 mRNAs (e.g. for keratin 14)
- Morphological appearance different than that of female glands
|
- Multiple sex-specific responses in gene expression to hormone exposure
- Greater levels of more than 1000 mRNAs (e.g. for thyroid hormone responsive SPOT14 homolog)
- Morphological appearance different than that of male glands
- Greater size of meibomian glands in upper lid
|
Mouse [6,8,90,117,118]
|
|
|
|
Cornea
|
|
|
- Thicker central, para-central and mid-peripheral zones of the epithelium
- Male donor transplants: better survival rates
- Higher horizontal diameter and sagittal height
- More scarring in keratoconus
- Higher levels of hundreds of mRNAs (e.g. epidermal growth factor receptor)
- Thicker central, para-central and mid-peripheral zones of the epithelium
- Greater epithelial mitotic index rate
|
- Shorter wetting time
- Better transplant recipient
- Greater hysteresis and resistance factor
- Higher sensitivity
- Higher endothelial cell density
- Higher prevalence of Salzmann nodular degeneration
- Oxybuprocaine anesthetic eye drops induce significant corneal thickness increases only in females
- Develop keratoconus at younger age
- Fungal ulcers re-epithelialize twice as slowly
- Higher levels of hundreds of mRNAs (e.g. transglutaminase 1)
- Lower mean dry-eye severity score in premenopausal women following LASIK with femtosecond laser and mechanical keratome
- Higher curvature
|
Human [9,15,23,119–154]
|
- Higher curvature
- Slower corneal epithelial wound closure and lower polymorphonuclear leukocyte responses
|
Mouse, dog, monkey [155–158]
|
|
|
|
Conjunctiva
|
|
|
- Higher goblet cell count
- Higher prevalence of allergic conjunctivitis in boys
- Higher prevalence of conjunctival inflammation in allergic rhinoconjunctivitis
- Higher prevalence of pterygium
- More distinct pinguecula
|
- Depression of goblet cell count around the time of ovulation
- More discomfort from pterygia
- Higher prevalence of superior limbic keratoconjunctivitis
|
Human[159?166]
|
|
|
|
Nasolacrimal duct
|
|
|
- Greater length, diameter of the bony nasolacrimal canal entrance and volume
|
- More frequent appearance of mucopeptide, bacterial and mixed concretions (dacryoliths) in the lacrimal drainage system
- Decreased aeration
- Higher prevalence of primary acquired nasolacrimal duct obstruction
|
Human [167–173]
|
|
|
|
Tear film
|
|
|
- Peak occurrence of short tear film breakup time in the third decade of life
- Thicker and less contaminated lipid layer (>45 years of age)
- Higher amounts of EGF, TGF-? and gender-specific tear protein
- Higher tear osmolarity (<41 years old)
- Higher 30 kDa caseinolytic activity
- Higher prevalence of dry eye symptoms in children (8.8 ± 3 years old)
|
- Peak occurrence of short tear film breakup time in the seventh decade of life
- Lower (non-invasive) tear breakup time, and an increase in tear osmolarity during aging
- Higher amounts of innate immune defense proteins
- Earlier decrease in tear peroxidase activity during aging
- Higher prevalence of dry eye
- Higher prevalence of dry eye disease after contact lens wear
- Higher prevalence of dry eye after LASIK
- Report of dry-eye symptoms more likely
|
Human [18,121,150,174–189]
|
- Higher tear levels of various proteins (e.g. SC, IgA, cystatin-related protein, TGF?, 42 kDa & 46 kDa proteins)
|
- Higher secretion and tear levels of various proteins (e.g. 20 kDa & 90 kDa proteins)
|
Mouse, rat, hamster [100–104,190,191]
|
Table 4 Summary of data on the major outcomes from the literature for most pain modalities in the laboratory setting, from Racine et al., 2012a [302]. back to top
|
Type of stimulus
|
Pain thresholda
|
Pain tolerancea
|
Pain intensity or unpleasantness
|
|
Cold pain
|
W = M
|
W < M
|
No consistent difference
|
|
Hot pain
|
No consistent difference
|
W < M
|
No consistent difference
|
|
Pressure pain
|
W < M
|
W < M
|
No consistent difference
|
|
Ischemic pain
|
W = M
|
W = M
|
No consistent difference
|
|
Muscle pain
|
No consistent difference
|
No consistent difference
|
No consistent difference
|
|
Chemical pain
|
No consistent difference
|
No consistent difference
|
No consistent difference
|
|
Electrical pain
|
No consistent difference
|
No consistent difference
|
No consistent difference
|
|
Visceral pain
|
No consistent difference
|
No consistent difference
|
No consistent difference
|
a Pain threshold refers to the least experience of pain that can be identified by a subject; Pain tolerance is defined as the highest level of pain that a subject is able to tolerate.
Table 5 Reported effects of orchiectomy or androgen treatment on the lacrimal glands of mice, rats, hamsters, guinea pigs and/or rabbits. back to top
|
Orchiectomy
|
Androgen treatment
|
|
Glandular degeneration
|
Reversal of the effects of orchiectomy on glandular structure, function & secretion
|
|
Decreased size of acinar cells & nuclei
|
Generation of many glycoprotein-secreting cells
|
|
Disappearance of vesicular mucus in acinar cells
|
Production of mucus & highly polymerized carbohydrates
|
|
Loss of cellular & nuclear polymorphism, & decreased nuclear volume
|
Appearance of PAS-positive material in acinar cells & central lumina
|
|
Fewer basophilic glandular cells & luminal enlargement
|
Induction of acinar cell & parenchymal hyperactivity
|
|
Alterations in levels of numerous mRNAs & proteins
|
Alteration in levels of thousands of mRNAs & numerous proteins
|
|
Attenuated alkaline phosphatase activity, & increase in N-acetyltransferase & hydroxyindole-o-methyl-transferase activity
|
Enlargement of glandular vesicles
|
|
Increased acinar epithelial cell susceptibility to cytomegalovirus infection
|
Reduction in total activity of cholinergic receptors
|
|
Proliferation of interfollicular connective tissue
|
Increase in total activity of Na+,K+-ATPase, acid phosphatase, alkaline phosphatase & β-adrenergic receptors
|
|
Decreased or increased harderianization
|
Suppression of glandular inflammation
|
|
Alteration in fluid or specific protein secretion
|
Change in fluid or specific protein secretion
|
|
Transformation of structure to neutral
(40 days after orchiectomy) or
female type morphology
|
Transformation of the glandular acino-serous structure into a “vesicular mucus” structure
|
Several of the responses listed in Table 5 are species- and/or strain-dependent [11–13,35,66,74,77–79,84,86–88,91,93,94,96,100,102–105,199,204,358–373,375–413]. In addition, older studies have reported no influence of orchiectomy or androgen treatment on the growth or histological characteristics of the lacrimal gland [416,417]. These latter findings may be attributed in part to differences in experimental design, variations in the age, sex, and endocrine status of animals, the dosage and time course of androgen administration, and the methods of analysis.
Table 6 Reported influence of estrogen treatment, ovariectomy, estrogen absence or anti-estrogen administration on the structure and function of the lacrimal gland in mice, rats, hamsters, rabbits and humans. back to top
|
|
Estrogen + (e.g. estrogen treatment, HRT)
|
Estrogen – (e.g. ovariectomy, estrogen absence, anti-estrogen administration)
|
|
Lacrimal gland regression
|
|
|
|
Lacrimal gland weight or morphology
|
no effect,
glandular appearance restored to a “female” type
|
no effect, morphological appearance changed to a “male” type
|
|
Alteration in structural profile to a “neutral” type
|
|
?
|
|
Lacrimal gland histology
|
|
no effect
|
|
Glandular tissue
|
|
|
|
Acinar cell disruption & vacuolization, necrosis
|
↓
|
, no effect
|
|
Connective tissue
|
|
↑
|
|
Lymphocyte infiltration
|
|
, no effect
|
|
DNA and RNA levels
|
↑
|
|
|
DNA degradation
|
↓
|
, no effect
|
|
Gene expression (numerous genes)
|
altered
|
altered
|
|
Leucine aminopeptidase amount
|
↑
|
|
|
PAS-staining
|
|
|
|
Total activity of β-adrenergic receptors
|
↑
|
|
|
Total activities of cholinergic receptors & Na+, K+-ATPase
|
|
|
|
Acid & alkaline phosphatase activities
|
|
|
|
Lacrimal fluid peroxidase
|
↑*, ↓*, no effect
|
|
|
Total protein content
|
no effect
|
altered
|
|
Membrane-bound protein content
|
|
altered
|
|
20 kDa protein content
|
|
altered
|
|
Specific protein secretion
|
no effect
|
|
|
Phenylephrine (α-adrenergic agonist)-induced peroxidase & total protein secretion
|
|
↑
|
|
Antagonism of certain androgen effects
|
?*
|
|
|
Inflammation
|
↓
|
no effect, ↑(SS)*
|
|
Tear output
|
↑* , no effect*
|
no effect, ↑(male only)
|
|
Dry eye improvement
|
?
|
|
|
Dry eye development
|
|
|
Check marks indicate that an effect of estrogen supplementation or absence was identified. Upward (↑) and downward (↓) arrows indicate direction of effect. Red text indicates negative impact on DED. Multiple entries signify conflicting results from different studies. Findings in humans are marked with an asterisk (*). SS, Sjögren Syndrome. Table adapted from Sullivan et al. [647] and updated [19,60,89,350,352,355,464,513,519,666,668–671,674–683].
Table 7 Effects of estrogen and progesterone supplementation on the ocular surface of postmenopausal women. back to top
|
Reference
|
Treatment (number of participants)
|
Duration
|
Administration
|
Control
|
Symptoms
|
TBUT
|
Schirmer test
|
Other parameters
|
|
Positive effect on dry eye
|
|
Akramian et al. [676]
|
E (n = 10)
|
4w
|
topical ointment
|
placebo
|
no effect
|
↑
|
↑
|
|
|
Sator et al. [675]
|
E + P (n = 42)
|
4 m
|
E topical drops + oral E + P
|
artificial tears* + oral E + P
|
↓(topical E group)
|
|
↑(topical E group)
|
|
|
Vavilis et al. [696]
|
E (n = 7) E + P (n = 4)
|
4 m
|
transdermal transdermal + oral
|
no control
|
|
|
|
improvement in conjunctival epithelium maturational changes
|
|
Affinito et al. [679]
|
E + P (n = 25)
|
3, 6 m
|
transdermal
|
untreated
|
↓
|
|
↑
|
|
|
Guaschino et al. [678]
|
E + P (n = 40)
|
12 m
|
oral
|
untreated
|
|
|
↑
|
|
|
Pelit et al. [697]
|
E + P (n = 17)
|
3 m
|
oral
|
no control
|
|
↑
|
no effect
|
↑ goblet cell density
|
|
Altintas et al. [677]
|
E + P (n = 15)
|
2 m
|
oral
|
untreated
|
|
↑
|
↑
|
|
|
Moon et al. [680]
|
E + P (n = 36)
|
1, 3 m
|
oral
|
no control
|
↓
|
|
↑
|
|
|
Coksuer et al. [681]
|
E + P (n = 34)
|
6 m
|
oral
|
no control
|
↓
|
↑
|
↑
|
|
|
Okon et al. [682]
|
HRT (n = 60)
|
3, 6, 12 m
|
oral
|
no control
|
|
↑
|
↑ (6, 12 m)
|
|
|
Negative effect on dry eye
|
|
Golebiowski et al. [689]
|
E (n = 10)
|
2 m
|
transdermal
|
placebo
|
↑
|
no effect
|
no effect
|
no effect on tear osmolarity, MG function, lid morphology, corneal or conjunctival sensitivity
|
|
Uncu et al. [674]
|
E (n = 5) E + P (n = 19)
|
6, 12 m
|
Transdermal oral
|
no control
|
|
|
↓ (12 m) worse E group
|
|
|
Erdem et al. [712]
|
E + P (n = 20)
|
3 m
|
oral
|
tear supplement
|
↑
|
no effect
|
no effect
|
possible effect on MG
|
|
Shaharuddin et al. [703]
|
E (n = 11) E + P (n = 19)
|
single time point
|
not stated
|
untreated
|
|
|
|
↑ frequency of dry eye signs
|
|
Schaumberg et al. [60]
|
HRT (n = 15681)
|
population study
|
various
|
untreated
|
|
|
|
↑ dry eye prevalence with HRT, worse with E only
|
|
Chia et al. [19]
|
HRT (n = 655)
|
population study
|
various
|
untreated
|
|
|
|
↑ dry eye prevalence with HRT
|
|
Equivocal effect on dry eye
|
|
Evans et al. [704]
|
E (n = 10)
|
single time point
|
transdermal or implant
|
untreated
|
↓
|
|
|
no difference in osmolarity
|
|
Kuscu et al. [707]
|
E + P (n = 10)
|
6 m
|
oral
|
no control
|
no effect
|
no effect
|
no effect
|
improved MG assessment, ↑tear IgA, no effect on tear lysozyme
|
|
Taner et al. [713]
|
E + P (n = 25)
|
6 m
|
oral
|
untreated
|
|
no effect
|
no effect
|
no effect on conjunctival cytology
|
|
Piwkumsribonruang et al. [708]
|
E + P (n = 21)
|
3 m
|
transdermal + oral
|
placebo
|
no effect
|
no effect
|
no effect
|
|
|
Jensen et al. [705]
|
HRT (n = 25)
|
single time point
|
various
|
untreated
|
↓
|
|
no effect
|
|
|
Lekskul et al. [714]
|
HRT (n = 38)
|
single time point
|
oral
|
untreated
|
|
no effect
|
no effect
|
|
Key: E, estrogen; P, progesterone; HRT, hormone replacement therapy (various constituents); TBUT, tear break up time; MG, meibomian gland; m, months; w, weeks. *benzalkonium chloride preserved. Table adapted from Truong et al. [350] and updated.
Table 8 Summary of IGF-1 effects on corneal wound healing. back to top
|
Model used
|
Epithelial cells/limbal stem cells (LSC)
|
Stroma/keratocytes
|
Endothelial cells
|
Innervation
|
|
Cell culture/signaling
|
- ↑ LSC differentiation [889].
- ↑ epithelial proliferation and migration, activating AKT and ERK [905].
- IGF-1R [906] and IGF-1R/IR hybrid nuclear localization [907].
- ↑ laminin-5 and beta-1 integrin [908]
- PKC and tyrosine kinase [909]
|
- ↑ human corneal fibroblast proliferation and collagen synthesis [884–887,911].
- Epi releases IGF-1 to act on stroma [912]
- ↑ Keratocyte migration [888]
|
- ↑ rabbit endothelial cell proliferation via IRS-1[893]
- ↑Bovine endothelial cell proliferation [891]
- No effect in cat endothelial cells [913]
|
|
|
|
|
Organ culture
|
- +Substance P: ↑ epithelial migration and healing [894–896]
|
|
↑ cell proliferation in human embryonic endothelial and stromal culture [892]
|
|
|
|
|
Animal model
|
- +Substance P: ↑ healing rat models of neurotrophic keratopathy [897,898] and diabetes [899] and rabbits [900]
- Preserves limbal stem cells in type II diabetic mice [890]
- No effect in a rat model of galactosemia [910]
|
|
|
↑ nerves in type II diabetic mice [890], LASIK rabbits [869]
|
|
|
|
Human study
|
+Substance P: Prevents SPK in diabetic patients post cataract surgery [901], accelerates reepithelialization in patients with neurotrophic keratopathy [902–904]
|
|
|
|
Table 9 Summary of insulin actions on corneal wound healing. back to top
|
Model used
|
Epithelial cells
|
Stroma/keratocytes
|
Endothelial cells
|
Innervation
|
|
Cell culture/signaling
|
- ↑ wound healing in the corneal epithelium by activating PI3K/AKT, ERK and EGFR [914]
- ↑ proliferation and anti-apoptotic [915]
- ↑ migration but not proliferation [916]
|
↑ bovine keratocyte matrix secretion, collagen synthesis [911,923,924]
|
- ↑ the Na, K-ATPase activity and pump function of cultured corneal endothelial cells, mediated by PKC [927]
- ↑ bovine and human endothelial cell proliferation [928,929]
|
|
|
|
|
Organ culture
|
↑ proteolysis in diabetic corneas [917]
|
|
|
|
|
|
|
Animal model
|
↑ Healing in streptozotocin rats [918–920]
|
|
|
Prevent nerve loss in streptozotocin-induced diabetic mice [930]
|
|
|
|
Human study
|
- Insulin receptor present on human ocular surface tissue in healthy and diabetic patients [921]
- ↑ auto-fluorescence and ↓ corneal sensitivity in type I diabetic patients [922]
|
Topical insulin not toxic to ocular surface [925,926]
|
|
|